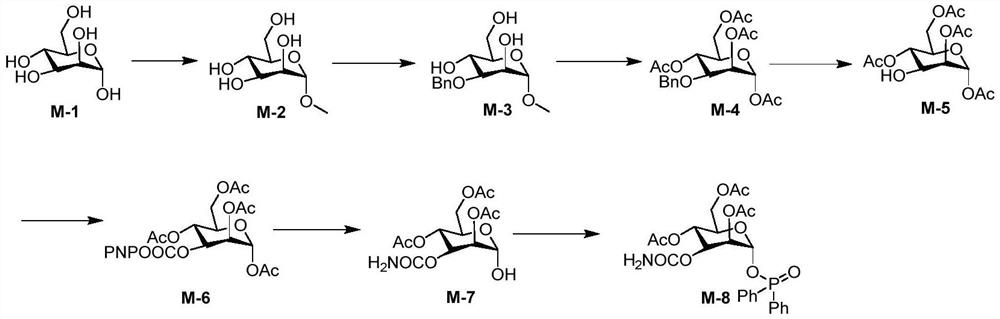
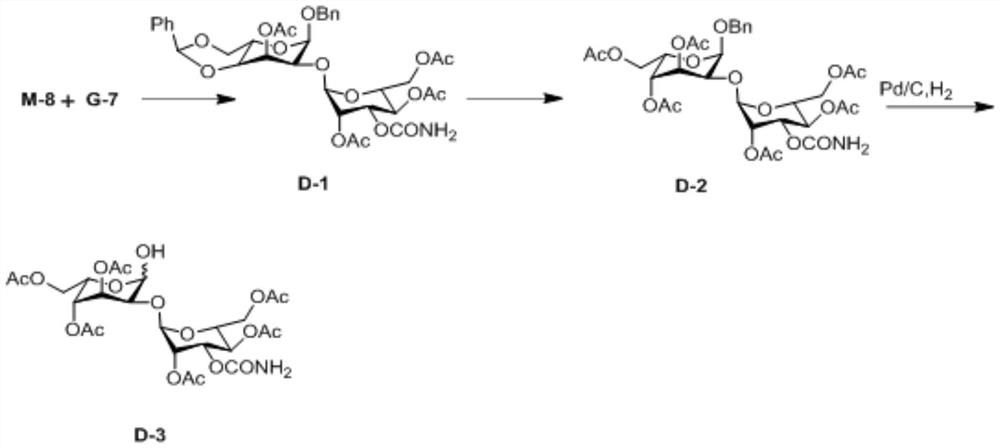
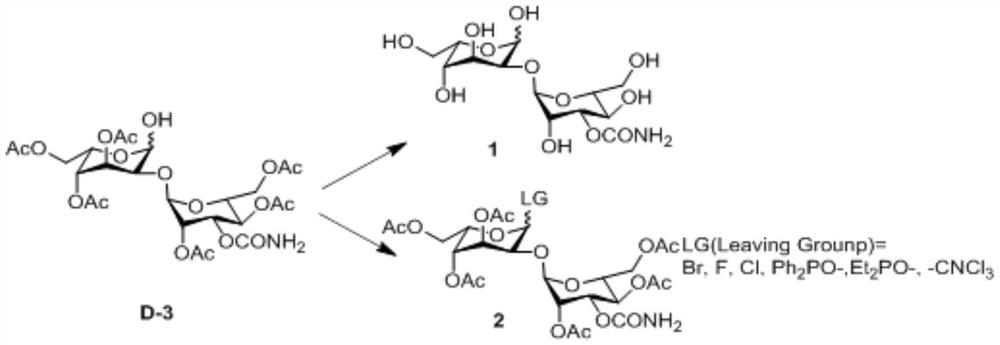
A kind of preparation method of 3-o-carbamoylmannose donor derivative, bleomycin disaccharide and its precursor
A technology for carbamoyl mannose and derivatives, which is applied in the field of medicine and chemical industry, can solve the problems of difficult purification, poor sorbitol selectivity, slow progress and the like, and achieves the effects of strong operability, cheap raw materials and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] An embodiment of the preparation method of the 3-O-carbamoylmannose donor derivative (M-8) of the present invention comprises the following steps:
[0053] (1) Preparation of methyl α-D-mannopyranoside (M-2)
[0054] The structural formula of the M-2 is:
[0055] Under the protection of nitrogen, 100 grams of mannose (M-1) (257.7mmoL) was dissolved in dry 250mL of methanol, 1.0mL of acetyl chloride was added, and after reflux for 6 hours, when the mannose was completely dissolved, the methanol was distilled off under reduced pressure to obtain The crude product was washed and filtered with absolute ethanol to obtain 188.2 g of methyl α-D-mannopyranoside as a white solid, with a yield of 97%.
[0056] 1 H NMR (400MHz, CDCl 3 )δ7.39-7.27(m,5H),4.72(s,1H),4.68-4.65(d,J=12.0Hz,1H),4.59-4.56(dd,J=12.0Hz,1H),4.03(t ,J=9.6Hz,1H),3.91(s,1H),3.81(d,J=8.0Hz,1H),3.72(d,J=12.8Hz,1H),3.66(d,J=9.2Hz,1H ), 3.53 (d, J=8.0Hz, 1H), 3.30 (s, 3H).
[0057] (2) Preparation of methyl...
Embodiment 2
[0082] An embodiment of the preparation method of the 3-O-carbamoylmannose donor derivative (M-8) of the present invention comprises the following steps:
[0083] (1) Preparation of methyl α-D-mannopyranoside (M-2)
[0084] Under the protection of nitrogen, 100 grams of mannose (M-1) (257.7mmoL) was dissolved in dry 250mL of methanol, 1.0mL of acetyl chloride was added, and after reflux for 6 hours, when the mannose was completely dissolved, the methanol was distilled off under reduced pressure to obtain The crude product was washed and filtered with absolute ethanol to obtain 188.2 g of methyl α-D-mannopyranoside as a white solid, with a yield of 97%.
[0085] (2) Preparation of methyl-3-O-benzyl-α-D-mannopyranoside (M-3)
[0086] Under the protection of nitrogen, 50 grams of M-2 (257.7mmoL) and 32 grams of dibutyltin oxide (129mmoL) were dissolved in 250mL of dry toluene, and after reflux for 1 hour, the toluene was evaporated under reduced pressure to obtain the crude prod...
Embodiment 3
[0098] An embodiment of the preparation method of 3-O-carbamoylmannose (M-8) of the present invention comprises the following steps:
[0099] (1) Preparation of methyl α-D-mannopyranoside (M-2)
[0100] Under the protection of nitrogen, 100 grams of mannose (M-1) (257.7mmoL) was dissolved in dry 250mL of methanol, 1.0mL of acetyl chloride was added, and after reflux for 6 hours, when the mannose was completely dissolved, the methanol was distilled off under reduced pressure to obtain The crude product was washed and filtered with absolute ethanol to obtain 188.2 g of methyl α-D-mannopyranoside as a white solid, with a yield of 97%.
[0101] (2) Preparation of methyl-3-O-benzyl-α-D-mannopyranoside (M-3)
[0102] Under the protection of nitrogen, 50 grams of M-2 (257.7mmoL) and 12.8 grams of dibutyltin oxide (51.6mmoL) were dissolved in 250mL of dry toluene, and after reflux for 1 hour, the toluene was evaporated under reduced pressure to obtain the crude product, which was dri...
PUM
| Property | Measurement | Unit |
|---|---|---|
| percent by volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com