Application of combination of benzoylaconitine and paeoniflorin to preparation of drug used for treating rheumatoid arthritis
A technology of benzoylaconitine and therapeutic drugs, which is applied in the field of medicine and can solve the problems of high toxic and side effects and affecting patients' medication compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
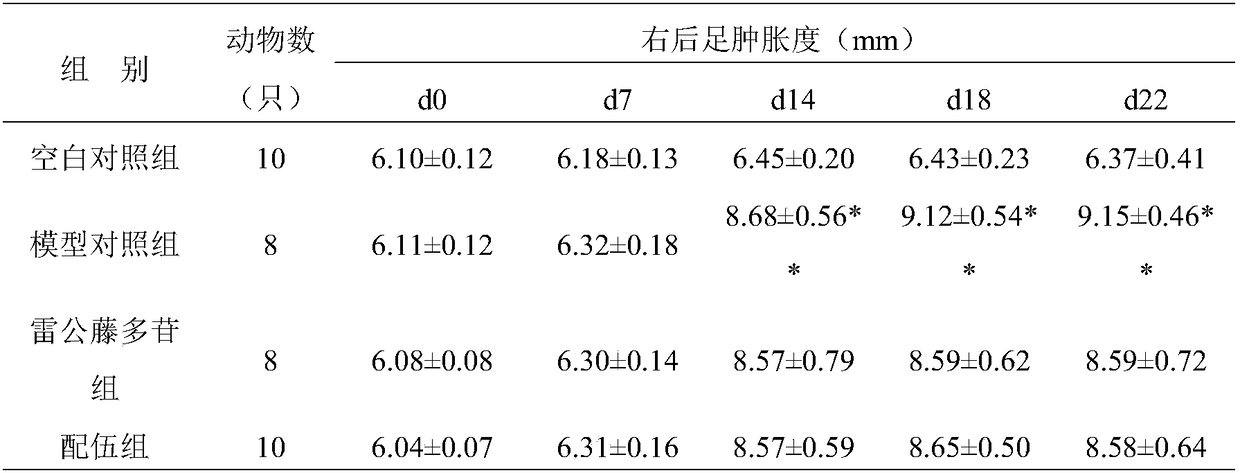
[0028] Study on the pharmacodynamic effect of benzoylaconitine combined with paeoniflorin on collagen-induced arthritis (CIA)
[0029] (1) Experimental animals
[0030] SD rats, male, weighing (140±10) g, were provided by the Institute of Experimental Animals, Sichuan Academy of Medical Sciences Sichuan Provincial People's Hospital, production license number: SCXK (Sichuan) 2013-15.
[0031] All experimental rats were adaptively fed for 5 days in the Experimental Animal Center of Sichuan Academy of Medical Sciences before the experiment. Breeding environment: temperature 22°C to 23°C, humidity 30% to 50%; feeding method: divided into cages according to groups, 5 birds per cage, free to eat and drink.
[0032] (2) Experimental drug and liquid preparation
[0033] (2.1) Experimental drugs
[0034] Benzoylaconitine, developed by Chengdu Mansite Biotechnology Co., Ltd. and Chengdu Institute of Biology, Chinese Academy of Sciences, purity: 99.96% (HPLC), batch number: MUST-16012...
Embodiment 2
[0093] Mechanism of benzoylaconitine combined with paeoniflorin in the treatment of collagen-induced arthritis
[0094] (1) experimental animals, (2) preparation of experimental medicine and medicinal liquid, same as in Example 1;
[0095] (3) Main experimental reagents
[0096] Bovine type II collagen solution (2mg ml-1), produced by Chondrex Company of the United States, batch number: SLBR1681V;
[0097] Complete Freund's Adjuvant (CFA), produced by Sigma, USA, batch number: 160346;
[0098] Rat interleukin 1β (IL-1β) ELISA kit, produced by Wuhan Elabscience, batch number: AK0016DEC23031;
[0099] Rat tumor necrosis factor α (TNF-α) ELISA kit, produced by Wuhan Elabscience, batch number: AK0016DEC23032
[0100] Rat VEGF ELISA kit, produced by Xinbosheng Biotechnology Co., Ltd., batch number R161221-103a
[0101] Rat immunoglobulin G (IgG) ELISA kit, produced by Wuhan Elabscience, batch number: AK0017JAN12020
[0102] Rat prostaglandin E2 (PGE2) ELISA kit, produced by Wu...
Embodiment 3
[0152] Embodiment 3 medicine embodiment
[0153] Take benzoylaconitine and paeoniflorin, according to the dosage ratio of 0.1-2.0:24 (unit: mg·kg -1 d -1 ) after dissolving, add other pharmaceutically acceptable ingredients, including excipients, excipients, etc., according to the existing pharmaceutical technology to make capsules, tablets, microcapsules, oral agents and / or injections, preferably Oral preparations or injections, thereby preparing a rheumatoid arthritis treatment drug.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com