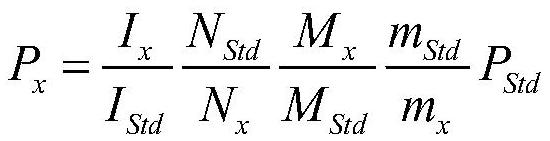A Method for Accurately Measuring the Purity of Volatile Substances Using Quantitative Proton Nuclear Magnetic Resonance Spectroscopy
A proton nuclear magnetic resonance spectroscopy and volatile technology, applied in the field of detection and analysis, can solve problems such as affecting the accuracy of measurement results, and achieve the effects of easy and accurate weighing, avoiding volatilization loss, and accurate measurement results.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
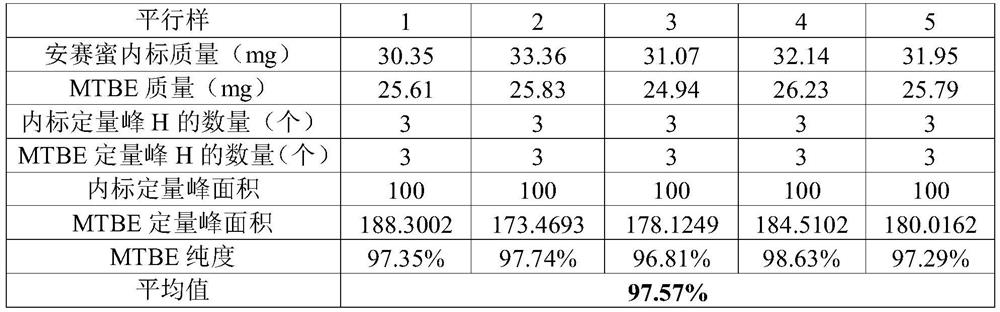
[0037] The purity of methyl tert-butyl ether (MTBE) was accurately measured by quantitative proton nuclear magnetic spectroscopy.
[0038] Target sample: methyl tert-butyl ether, a colorless, low-viscosity liquid, slightly soluble in water, miscible with many organic solvents, with a boiling point of 55°C, molecular formula C 5 h 12 O, the molar mass is 88.15. The sample was purchased from Sigma Company, and the gas chromatography (GC) purity was 99.94%.
[0039] At first adopt routine technology to measure: internal standard is the commonly used quantitative NMR solid internal standard acesulfame potassium (GBW (E) 100065, purity 99.6%, expanded uncertainty 0.6%, k=2), molecular formula C 4 h 4 KNO 4 S, the molar mass is 201.24.
[0040] Weigh about 32mg of acesulfame potassium solid internal standard into a 1.5mL solution bottle, weigh the mass of the internal standard, accurate to 0.01mg; use a pipette to pipette about 25mg of methyl tert-butyl ether, add the solution ...
Embodiment 2
[0050] The purity of tert-amyl methyl ether (TAME) was accurately measured by quantitative proton nuclear magnetic spectroscopy.
[0051] Target sample: methyl tert-amyl ether, boiling point 85°C, molecular formula C 6 h 14 O, the molar mass is 102.18. The sample was purchased from Sigma Company, and the gas chromatography (GC) purity was 98.60%.
[0052] Adopt the method of the present invention, the internal standard selects national primary standard substance ethyl acetate (GBW06114, purity 99.7%, expanded uncertainty 0.4%, k=2), boiling point 77.5 ℃, molecular formula C 4 h 8 o 2 , with a molar mass of 88.11.
[0053] Transfer 0.6mL of deuterated chloroform to a 1.5mL sealed sample bottle with a 1000 μL airtight needle, transfer about 19 mg of internal standard ethyl acetate into the sample bottle with a 50 μL airtight needle, and use a high-precision balance (d = 0.01 mg) weigh the mass of ethyl acetate; then use another 50 μL airtight needle to transfer about 15 mg...
Embodiment 3
[0058] The purity of isopropanol (IPA) was accurately measured by quantitative proton nuclear magnetic spectroscopy.
[0059] Target sample: Isopropanol, a colorless transparent liquid with an odor like a mixture of ethanol and acetone. Soluble in water, also soluble in alcohol, ether, benzene, chloroform and most other organic solvents. Boiling point is 82°C, molecular formula C 3 h 8 O, the molar mass is 60.1. The sample was purchased from Sigma Company, and the gas chromatography (GC) purity was 99.98%.
[0060] Adopt the method of the present invention, the internal standard selects national primary standard substance methanol (GBW06111, purity 99.7%, expanded uncertainty 0.3%, k=2), boiling point 65.4 ℃, molecular formula CH 4 O, the molar mass is 32.04.
[0061] Transfer 0.6mL deuterated dimethyl sulfoxide to a 1.5mL sealed sample vial with a 1000 μL airtight needle, transfer about 29 mg of internal standard methanol into the sample vial with a 50 μL airtight needle...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com