Method for preparing resveratrol
A technology of resveratrol and compounds, which is applied in the field of synthesis of organic compounds, can solve the problems of serious environmental pollution and expensive raw materials, and achieve the effects of high reaction rate, low price and convenient processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
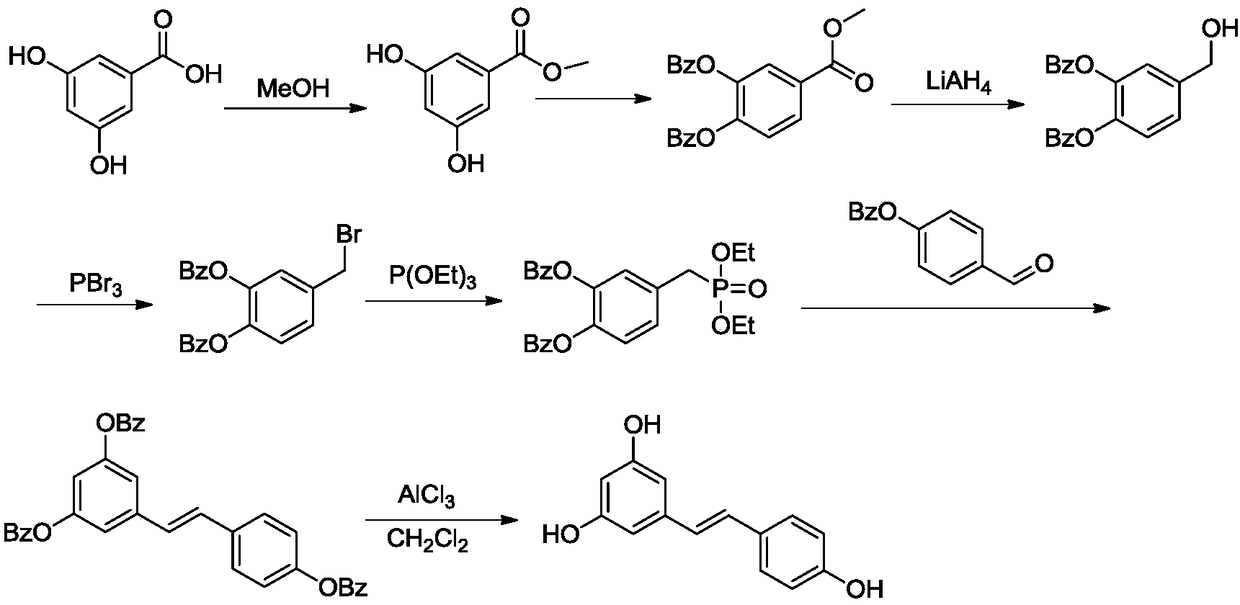
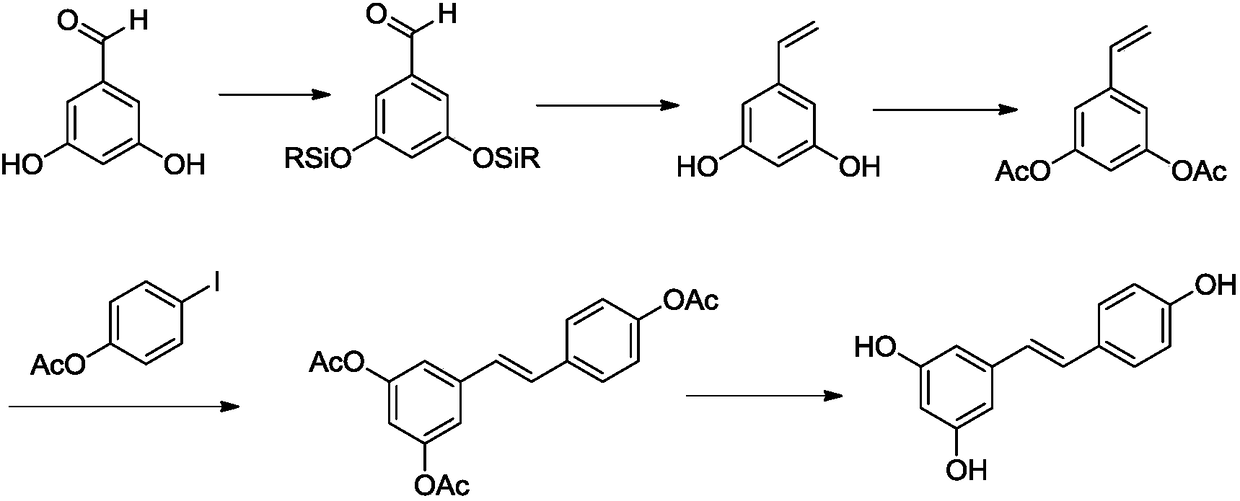
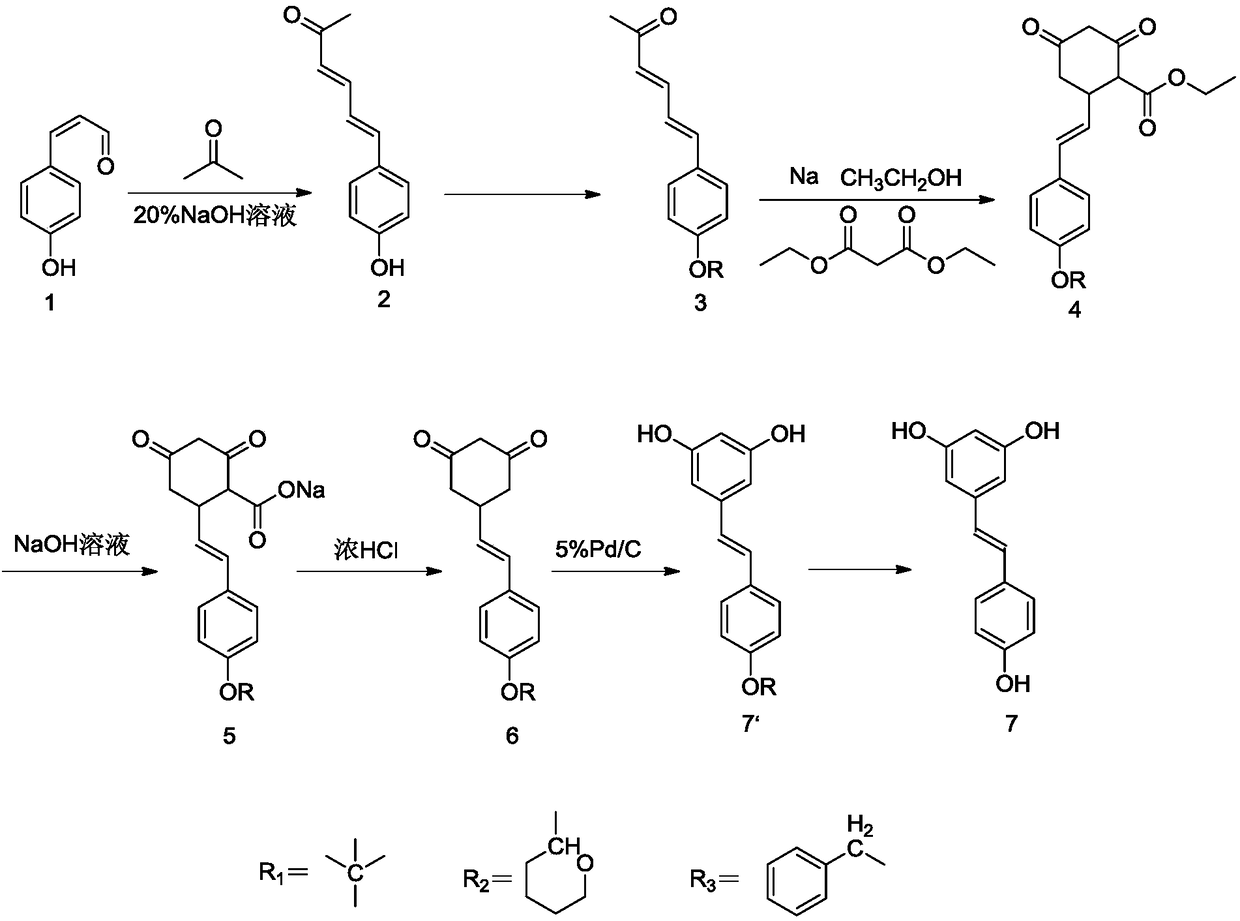
[0039] The synthetic route of resveratrol (1) is:
[0040]
[0041] Example 1
[0042] The synthetic method of resveratrol (7), the steps are as follows:
[0043] 1) Preparation of 6-[4-hydroxyphenyl]-3,5-hexadien-2-one (compound 2)
[0044] Mix 1 g of p-hydroxycinnamaldehyde, 10 ml of acetone, and 2 ml of 20% sodium hydroxide solution, and stir at room temperature for 3 h. Add water to precipitate a solid, filter and wash with water to obtain the product 6-[4-hydroxyphenyl]-3,5-hexadien-2-one with a yield of over 95%.
[0045] The experimental data are: 1H NMR (400MHz, dmso) δ9.83(s, 1H), 7.42–7.29(m, 2H), 7.00(d, J=15.5Hz, 1H), 6.89(d, J=10.6Hz ,1H),6.77–6.72(m,2H),6.13(d,J=15.5Hz,1H),2.21(s,3H).
[0046] 2) Preparation of 6-[4-phenylbenzyloxy]-3,5-hexadien-2-one (compound 3)
[0047] Mix 1 g of 6-[4-hydroxyphenyl]-3,5-hexadien-2-one, 12 ml of tetrahydrofuran, 0.54 g of potassium carbonate, and 0.72 g of benzyl bromide, stir at reflux at 70°C for 3 h, and quench with...
Embodiment 2
[0062] 1) Preparation of 6-[4-hydroxyphenyl]-3,5-hexadien-2-one (compound 2)
[0063] Mix 5g of p-hydroxycinnamaldehyde, 40ml of acetone, and 9ml of 20% sodium hydroxide solution, and stir at room temperature for 3h. Add water to precipitate a solid, and wash with suction to obtain 6-[4-hydroxyphenyl]-3,5-hexadien-2-one with a yield of over 98%.
[0064] The experimental data are: 1H NMR (400MHz, dmso) δ9.83(s, 1H), 7.42–7.29(m, 2H), 7.00(d, J=15.5Hz, 1H), 6.89(d, J=10.6Hz, 1H), 6.77–6.72(m, 2H), 6.13(d, J=15.5Hz, 1H), 2.21(s, 3H).
[0065] 2) Preparation of 6-[4-phenylbenzyloxy]-3,5-hexadien-2-one (compound 3)
[0066] Mix 5g of 6-[4-hydroxyphenyl]-3,5-hexadien-2-one, 5ml tetrahydrofuran, 3g potassium carbonate and 3.8g benzyl bromide, stir at reflux at 70°C for 3h, add water to quench, and the column layer The p-benzyloxycinnamaldehyde was obtained by analysis, and the yield reached more than 95%.
[0067] The experimental data is: 1 H NMR (400MHz, dmso) δ7.51 (d, J = 8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com