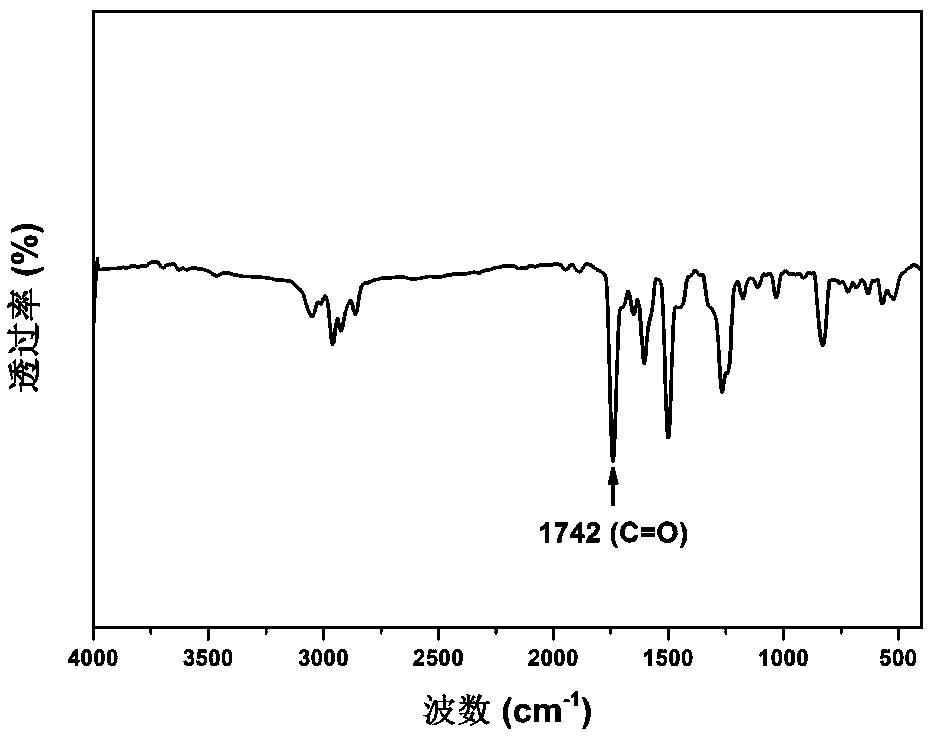
Diphenol monomer containing asymmetric fluorophore structure as well as preparation method and application thereof
A fluorophore, asymmetric technology, applied in the field of electrochromism and electronically controlled fluorescence, can solve the problems of unstable electrical activity, slow, low fluorescence contrast development of electronically controlled fluorescent materials, etc., to reduce the degree of aggregation quenching and accumulation Action, effect of electrochromic and electroluminescent performance enhancement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] The invention provides a preparation method of the diphenol monomer containing an asymmetric fluorophore structure, comprising the following steps:
[0043] 4-fluoronitrobenzene, R 2 -NH 2 , triethylamine and dimethyl sulfoxide are mixed, and a nucleophilic substitution reaction occurs to obtain a compound having a structure shown in formula II;
[0044] The compound having the structure shown in formula II, copper powder, potassium carbonate, 18-crown-6, R 1 -X is mixed with o-dichlorobenzene, and Ullmann reaction I occurs to obtain a compound with the structure shown in formula III; the R 1 X in -X is Cl, Br or I;
[0045] mixing the compound having the structure shown in formula III, Pd / C, hydrazine hydrate and dioxane, and performing a reduction reaction to obtain the compound having the structure shown in formula IV;
[0046] Mix the compound with the structure shown in formula IV, p-iodoanisole, copper powder, 18-crown-6, potassium carbonate and o-dichlorobenz...
Embodiment 1
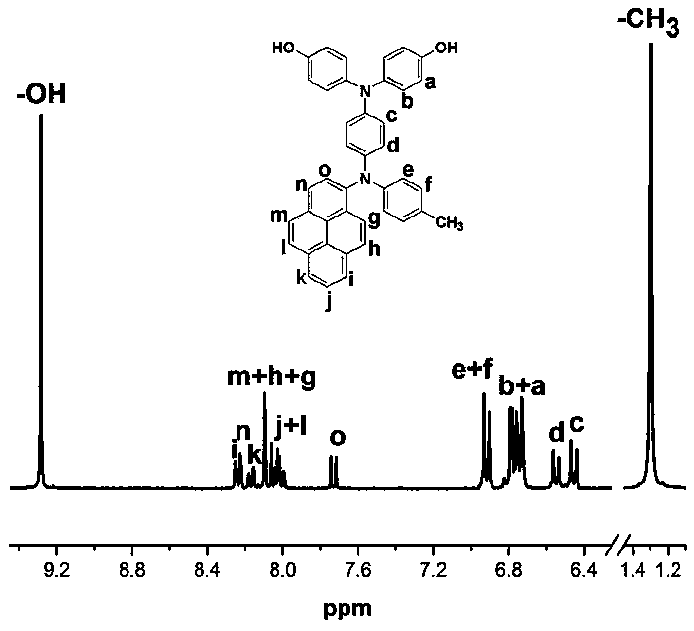
[0144] The preparation of N,N-bis(4-hydroxyphenyl)-N'-4-methylphenyl-N'-1-aminopyrene-1,4-phenylenediamine, its structural formula is as follows:
[0145]
[0146] Under nitrogen, add 16.9g (120mmol) of 4-fluoronitrobenzene, 16.7g (156mmol) of p-methylaniline, 15.8g (156mmol) of triethylamine in a 250mL three-necked flask equipped with mechanical stirring , adding 130 mL of dimethyl sulfoxide as a solvent, and reacting at 85° C. for 36 h. Discharge in ice-water mixture at room temperature, filter with suction, and recrystallize the filter cake with ethanol and N,N-dimethylformamide to obtain 21.9 g of 4-nitro-4'-methyldiphenylamine with a yield of 80% ;
[0147] Under nitrogen, add 10.0 g (35.6 mmol) of 1-bromopyrene, 9.0 g (37.4 mmol) of 4-nitro-4'- Copper powder of methyl diphenylamine, 9.0g (142.2mmol), 19.6g (142.2mmol) of potassium carbonate, 4.7g (17.8mmol) of 18-crown-6 are raw materials, add 60mL of o-dichlorobenzene as solvent, 160 Reaction at ℃ for 18h; Suction...
Embodiment 2
[0153] The preparation of N,N-bis(4-hydroxyphenyl)-N'-9-anthracenyl-N'-2-aminoanthracene-1,4-phenylenediamine, its structural formula is as follows:
[0154]
[0155] Under nitrogen, add 7.1g (50.0mmol) of 4-fluoronitrobenzene, 14.5g (75.0mmol) of 2-aminoanthracene, 7.6g (75.0mmol) of Triethylamine was then added to 58.9 mL of dimethyl sulfoxide, and reacted at 95°C for 42h. Discharge into ice-water mixture at room temperature, filter with suction, and recrystallize the filter cake with ethanol and N,N-dimethylformamide to obtain 12.9 g of 4-nitrophenyl-2-aminoanthracene with a yield of 82%;
[0156] Under nitrogen, add 7.8g (30.3mmol) of 9-bromoanthracene, 12.0g (36.3mmol) of 4-nitrophenyl-2 -Aminoanthracene, 9.6g (151.5mmol) copper powder, 20.9g (151.5mmol) potassium carbonate, 5.6g (21.2mmol) 18-crown-6, then add 69.4ml o-dichlorobenzene, react at 155°C for 14h ; Suction filtration while it was hot, distilled o-dichlorobenzene under reduced pressure, the resulting crud...
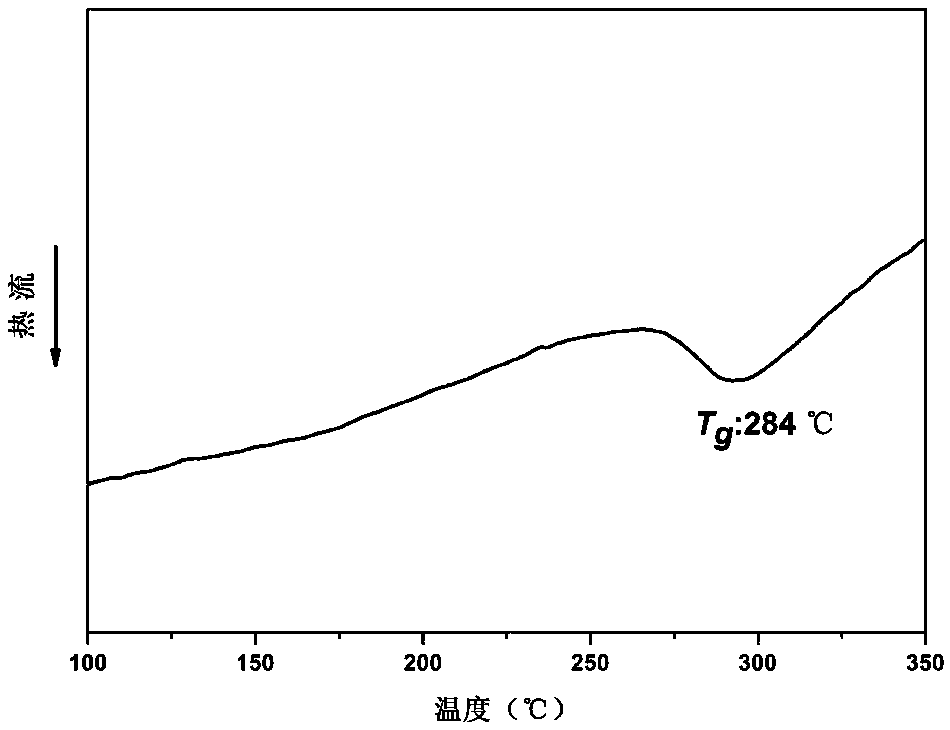
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com