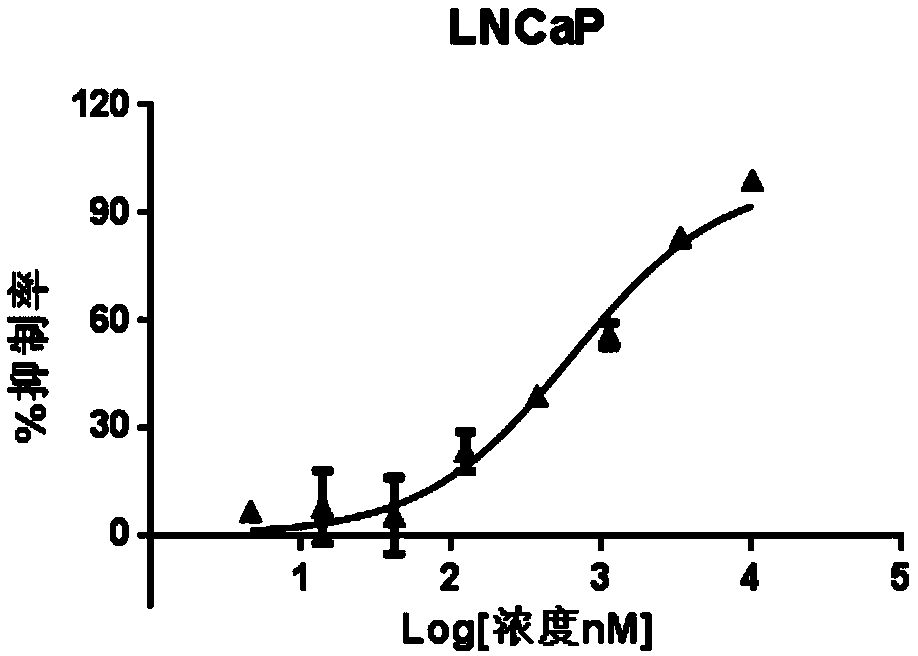
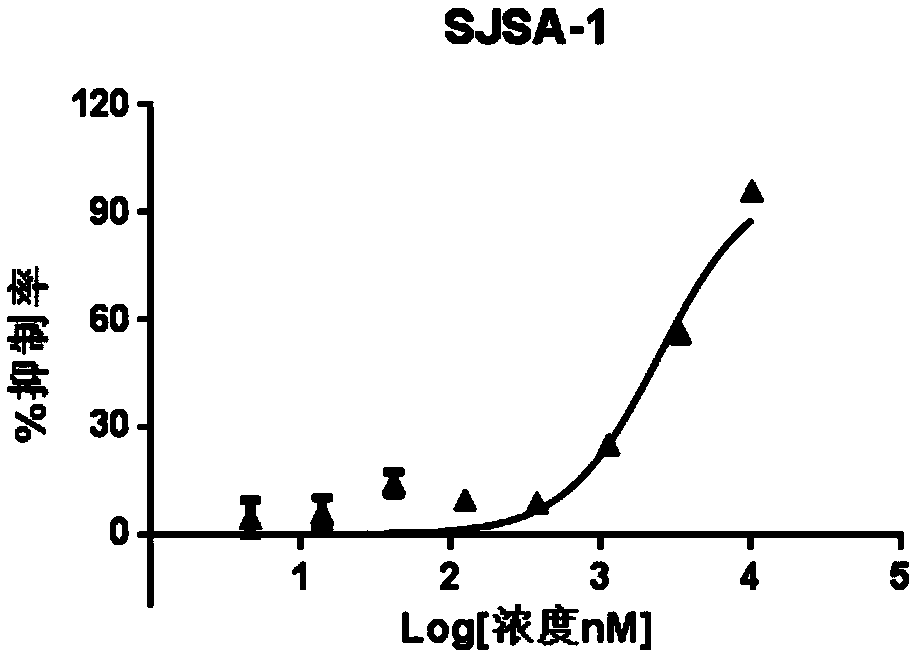
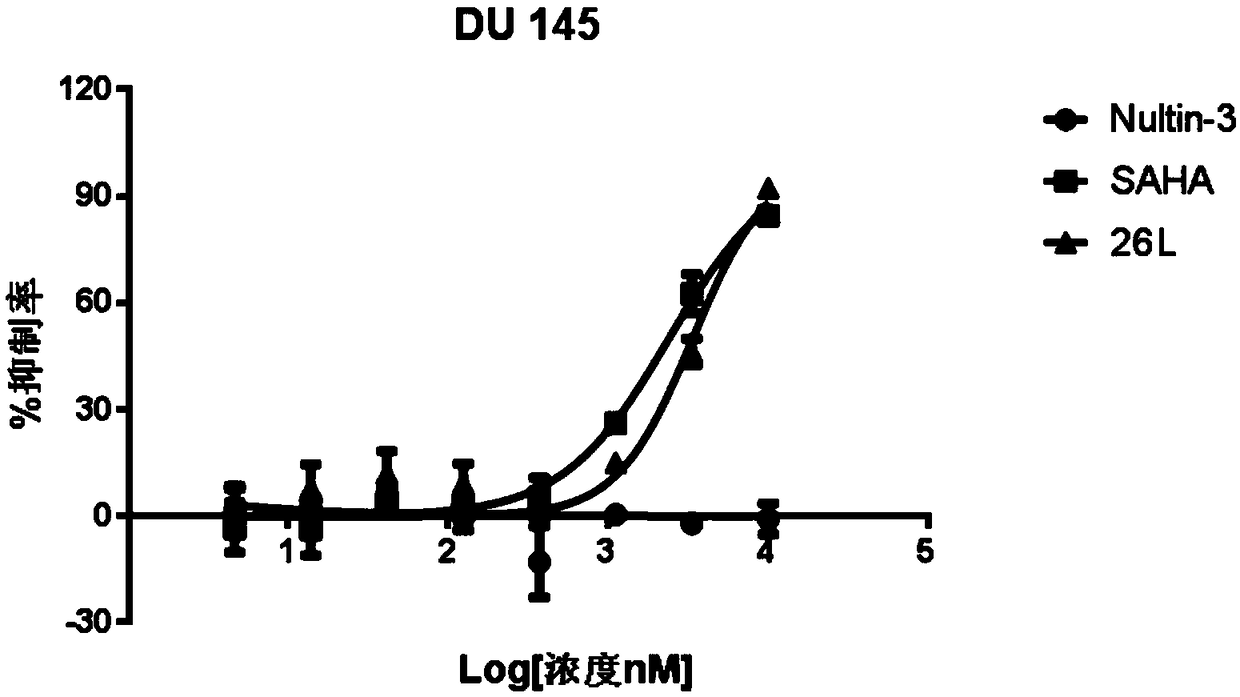
MDM2-HDAC double-target inhibitor, medicinal composition and preparation and application of MDM2-HDAC double-target inhibitor
An MDM2-HDAC, dual-target technology, applied in drug combinations, active ingredients of heterocyclic compounds, antipyretics, etc., can solve the problems of p53 activity inhibition and proportion imbalance, and achieve inhibition of ubiquitination and reduction of drug resistance. , the effect of increasing clinical benefit
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0127]
[0128] Dissolve compound 1 (62mmol) and compound 2 (60mmol) in 200ml methanol, add piperidine (12mmol) after fully dissolved, and reflux at 70°C. After 5 hours, cool to room temperature and stir overnight. After the reaction was complete, the suspension was filtered, and the resulting solid was washed three times with methanol and then dried to obtain a yellow solid 3. Data for Compound 3: 1 H NMR (500MHz, DMSO-d 6 )δ7.76–7.72(m,1H),7.67(td,J=2.8,5.5Hz,1H),7.64(s,1H),7.57–7.54(m,2H),7.39(d,J=8.2Hz ,1H),6.94(dd,J=2.0,8.2Hz,1H),6.90(d,J=1.9Hz,1H); 13 C NMR (125MHz, DMSO) δ168.76, 144.95, 136.89, 135.32, 134.97, 134.02, 131.21, 130.01, 129.39, 128.18, 128.10, 124.13, 121.54, 119.99, 110.71;
[0129]
[0130] Compound 4 (1eq) was dissolved in ultra-dry toluene and compound 5 (1.1eq) and 6 (1.2eq) were added sequentially, stirred at 70°C for 5h, cooled to room temperature after the reaction was complete, concentrated by filtration, silica gel column chromatograph...
Embodiment 2
[0208]
[0209] After compound 28 (30mmol) and compound 29 (29mmol) were fully dissolved in 150ml of methanol, a methanol solution of sodium methoxide (25wt%, 10mL, 44mmol) was slowly added dropwise. Heat and stir at 50°C for 3 hours. The mixture became cloudy, cooled to room temperature and filtered. After washing with water and ice methanol three times, the filter cake was vacuum-dried to obtain 6.4 g of compound 30 as a white solid. 1 H NMR (400MHz, Chloroform-d) δ7.88–7.79(m,2H), 7.66–7.59(m,2H), 7.49–7.42(m,5H).
[0210]
[0211] Compound 31 (2.71 g, 20.0 mmol) and compound 32 (21.0 mmol) were dissolved in 50 ml of dichloromethane solution and stirred overnight at room temperature. After the reaction was completed, the reaction solution was concentrated under vacuum to obtain 4.4 g of compound 33, which was used in the next step without further purification. ESI-MS(m / z):427(2M+H) +
[0212]
[0213] Compound 30 (7.3mmol) and compound 33 (7.3mmol) were dissol...
Embodiment 3
[0234]
[0235] Dissolve compound 39 (100mg, 0.536mmol) and compound 40 (230mg, 1.072mmol) in 3mL of toluene, add palladium acetate (2.4mg, 0.01072mmol) and potassium tert-butoxide (138mg, 1.233mmol) successively, and reflux reaction overnight . After fully cooling, add 6mL of petroleum ether to the system, filter, wash with petroleum ether and toluene respectively, and concentrate. After rough column chromatography purification, add 1,4-dioxane hydrochloric acid solution and stir for 1 hour, and concentrate to obtain a white solid. Compound 42. ESI-MS m / z 265(M+H) + .
[0236]
[0237] Compound 35 (70mg, 0.16mmol) and 2mL DCM were added to a 25mL round-bottomed flask. After fully dissolving, HATU (93mg, 0.24mmol) and DIEA (0.48mmol) were added successively. After stirring for 10 minutes, compound 42 (0.19mmol) was added. React overnight at room temperature. After the reaction was completed, it was extracted, dried and concentrated, purified by column chromatography ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com