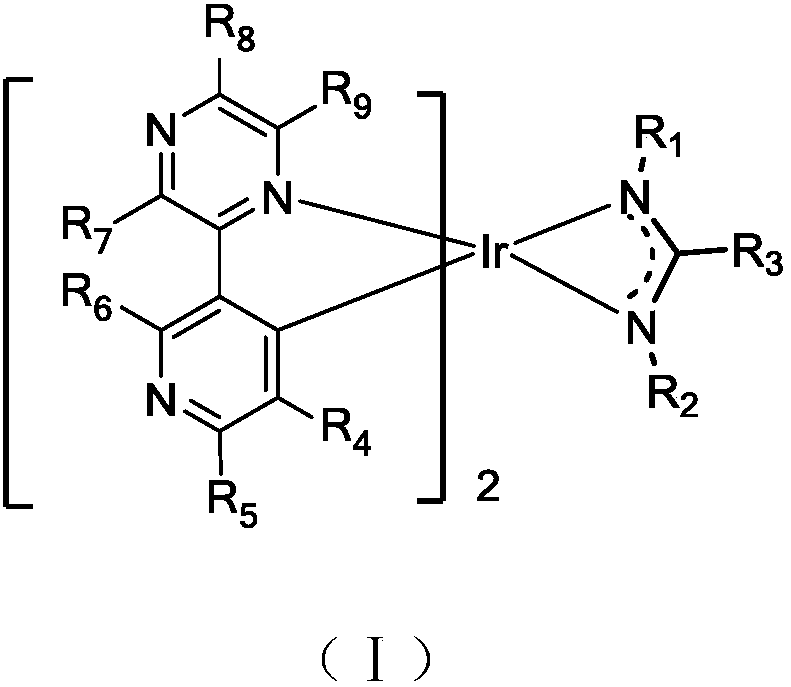
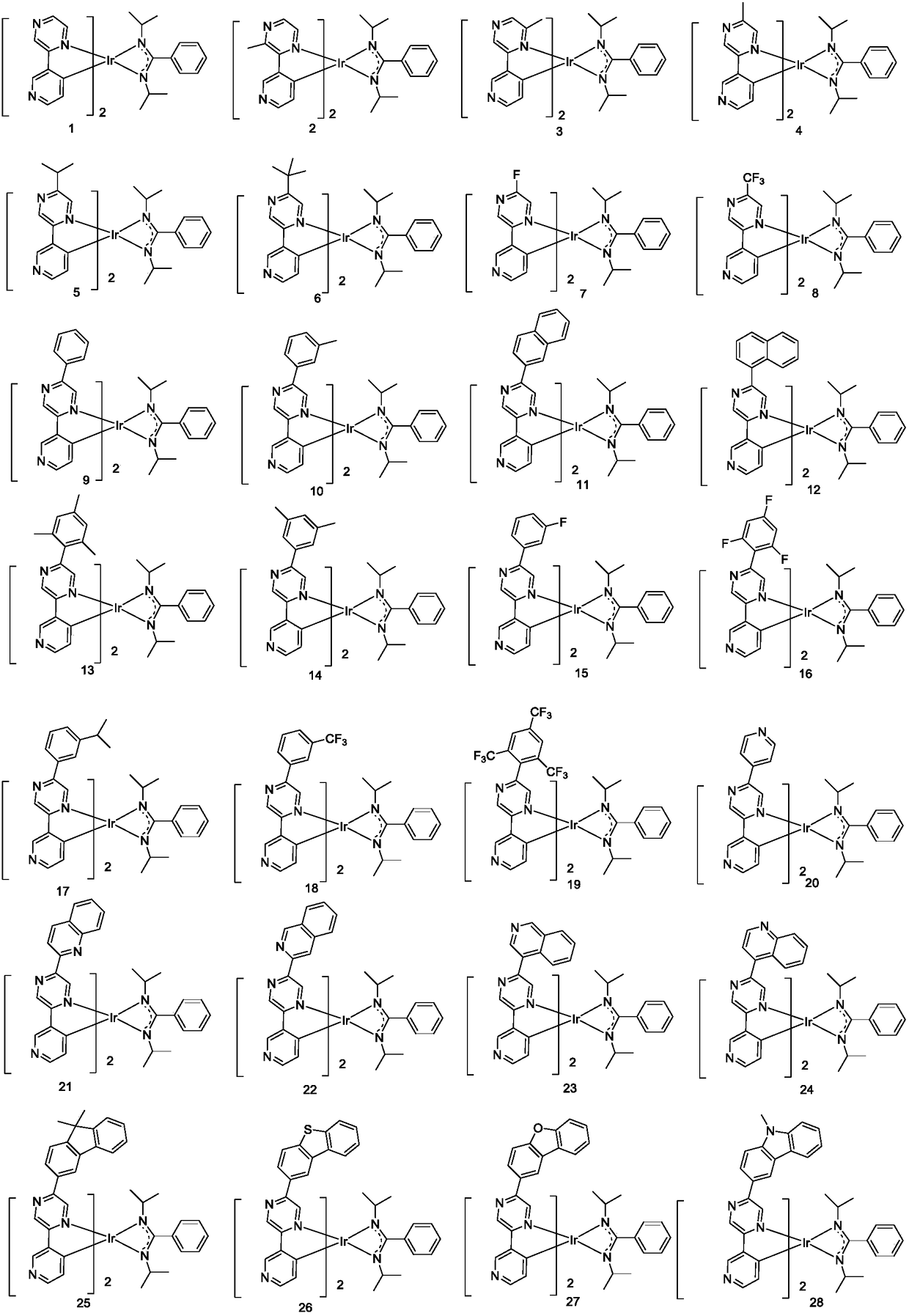
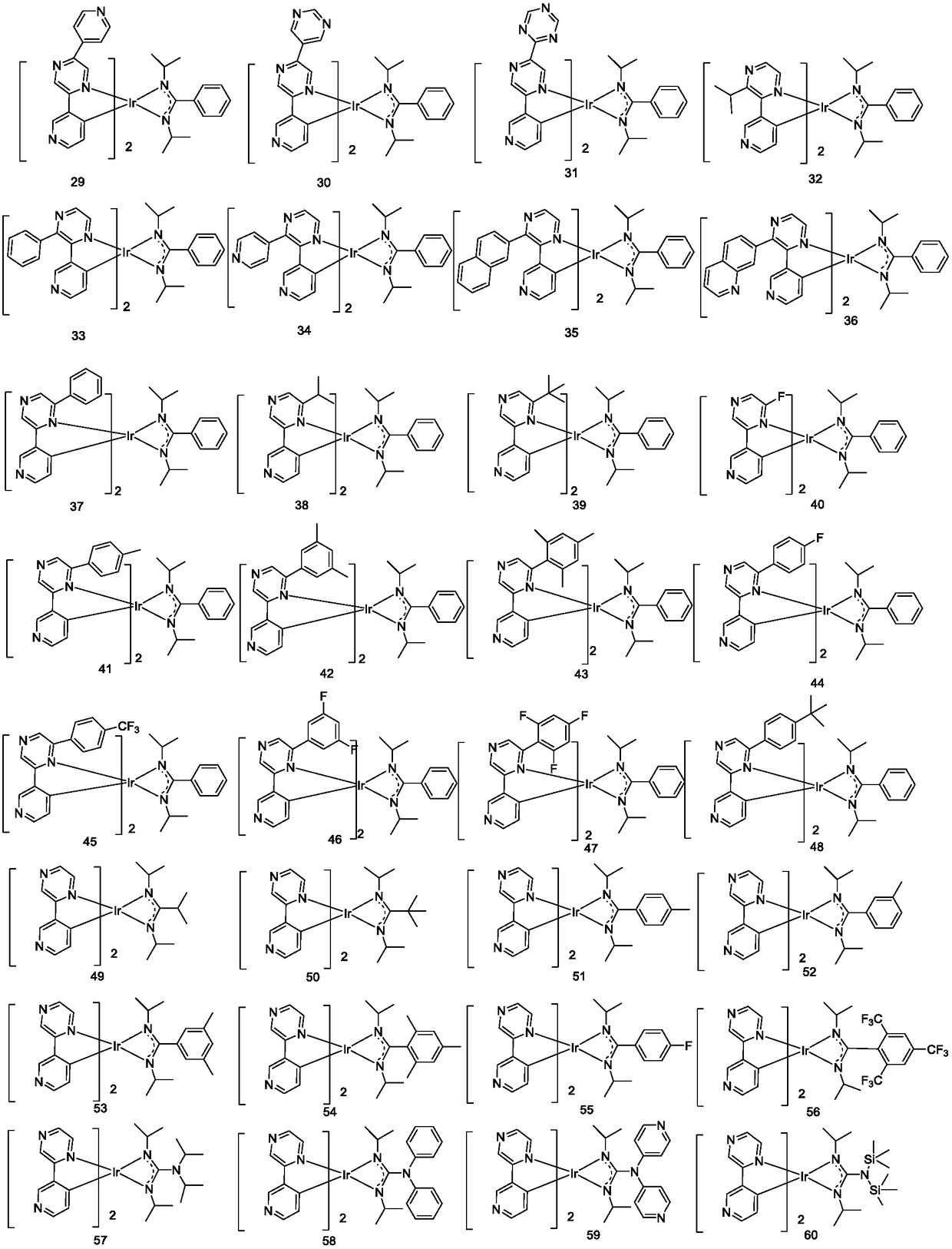
Iridium metal organic complex and organic light-emitting device thereof
A metal-organic and complex technology, used in indium organic compounds, platinum group organic compounds, luminescent materials, etc., can solve the problem that high-performance red phosphorescent materials need to be further developed, and meet the needs of industrialization. The effect of easy availability of film and raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Example 1: Preparation of Compound 1
[0066]
[0067] Preparation of Intermediate A1
[0068] 6.0 ml (12.0 mmol) of lithium diisopropylamide (LDA) was added to 50 ml of diethyl ether. At -78°C, 0.91 ml (10.0 mmol) of difluoropyridine was added dropwise, and the mixture was stirred for one hour. 1.4 ml (12.5 mmol) of trimethyl borate was added to the resulting mixture and stirred at room temperature for one hour.
[0069] After the reaction was completed, 20 mL of a 5% aqueous NaOH solution was added to the reaction mixture. The reaction mixture was separated into an organic layer and an aqueous layer. The aqueous layer was neutralized with 3M aqueous HCl. The neutralized aqueous layer was extracted with an additional 20 ml of ethyl acetate. The extraction process was repeated three times to obtain an organic layer. The organic layer was MgSO 4 dry. Compound b was obtained (1.03 g, 65%).
[0070] 570 mg of compound b and 0.4 ml of 2-bromo-4-methylpyridine (c...
Embodiment 2
[0076] Example 2: Preparation of Compound 4
[0077] The c1 in Example 1 was replaced with an equimolar amount of c4, and other steps were the same as those in the synthesis of Example 1 to obtain the target product compound 4 (4.42 g, 30%).
[0078]
[0079] Mass spectrum m / z: 736.28 (calcd: 736.27). Theoretical element content (%) C 33 H 36 IrN 8 : C, 53.79; H, 4.92; Ir, 26.08; N, 15.21 Measured element content (%): C, 53.79; H, 4.91; Ir, 26.08; N, 15.22. The above results confirmed that the obtained product was the target product.
Embodiment 3
[0080] Example 3: Preparation of Compound 9
[0081] The c1 in Example 1 was replaced with an equimolar amount of c9, and other steps were the same as in the synthesis of Example 1 to obtain the target product compound 9 (4.99 g, 29%).
[0082]
[0083] Mass spectrum m / z: 861.31 (calcd: 861.30). Theoretical element content (%) C 43 H 40 IrN 8 : C, 59.98; H, 4.68; Ir, 22.32; N, 13.01 Measured element content (%): C, 59.98; H, 4.69; Ir, 22.32; N, 13.00. The above results confirmed that the obtained product was the target product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com