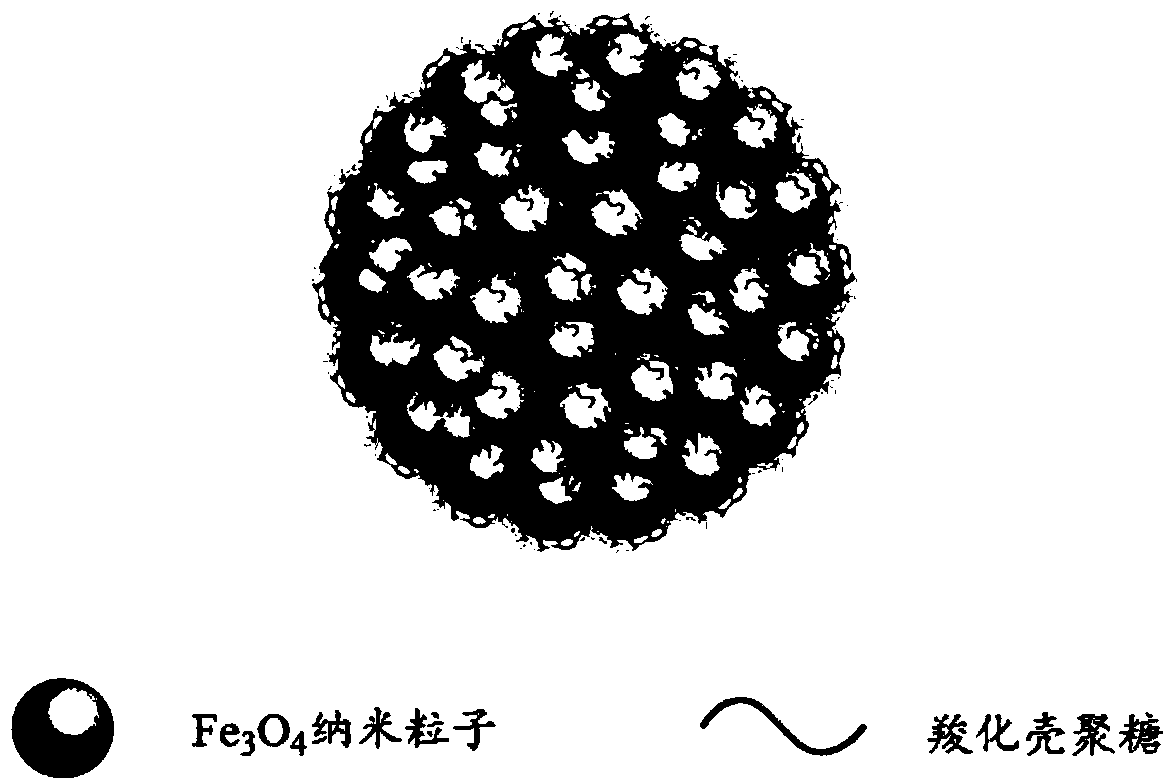
Preparation method of superparamagnetic carboxylated chitosan/Fe3O4 nanoparticle aggregate
A carboxylated chitosan, superparamagnetic technology, applied in the preparations for in vivo experiments, medical preparations with inactive ingredients, and medical preparations containing active ingredients, etc., can solve the problem of single superparamagnetic nanoparticles. Low magnetic responsiveness, difficult magnetic responsiveness and lateral relaxation rate of a single magnetic particle, etc., to achieve the effect of simple and efficient preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] 6.33g FeCl 3 ·6H 2 O and 2.33 g FeCl 2 4H 2 O is dissolved in 100g deionized water, add 0.67g carboxylated chitosan (carboxyl substitution degree 0.8, viscosity average molecular weight about 200,000), heat up to 60°C, after the carboxylated chitosan is completely dissolved, under the speed of 100rpm , adding 10g of concentrated ammonia water, the system immediately formed a black precipitate, after 30 minutes of heat preservation, the reaction temperature was raised to 90°C, and the heat preservation was continued for 45 minutes to obtain Fe 3 o 4 Aqueous dispersion of nanoparticles. The whole reaction was carried out under nitrogen atmosphere. After the reaction, the precipitate was washed with deionized water until the conductivity of the dispersion reached 10 μS cm -1 the following. After concentration, Fe 3 o 4 Fe in the aqueous dispersion of nanoparticles 3 o 4The mass fraction of nanoparticles was increased to 5%.
[0037] Weigh 2.5 g of emulsifier Sp...
Embodiment 2
[0043] 8.1g Fe 2 (SO 4 ) 3 and 5.6g FeSO 4 Dissolve in 130g deionized water, add 6.5g carboxylated chitosan (the degree of carboxyl substitution is 0.65, the viscosity average molecular weight is about 300,000), heat up to 65°C, after carboxylated chitosan is completely dissolved, under the rotating speed of 600rpm, Add 21.6g of ammonia water, the system immediately forms a black precipitate, after 100 minutes of heat preservation, the reaction temperature is raised to 95°C, and the heat preservation is continued for 30 minutes to obtain Fe 3 o 4 Aqueous dispersion of nanoparticles. The whole reaction was carried out under nitrogen atmosphere. After the reaction, the precipitate was washed with deionized water until the conductivity of the dispersion reached 10 μS cm -1 the following. After concentration, Fe 3 o 4 Fe in the aqueous dispersion of nanoparticles 3 o 4 The mass fraction of nanoparticles was increased to 10%.
[0044] Weigh 1.5g Tween-80 and 1.5g Span-8...
Embodiment 3
[0050] 18.4g FeCl 3 ·6H 2 O and 11.5g Fe(BF 4 ) 2 ·6H 2 O is dissolved in 80g deionized water, add 4.6g carboxylated chitosan (carboxyl substitution degree 0.95, viscosity average molecular weight is about 80,000), heat up to 70°C, after carboxylated chitosan dissolves completely, under the rotating speed of 500rpm , add 22.9g of ammonia water, the system immediately forms a black precipitate, after 60min of heat preservation, the reaction temperature is raised to 85°C, and the heat preservation is continued for 60min to obtain Fe 3 o 4 Aqueous dispersion of nanoparticles. The whole reaction was carried out under nitrogen atmosphere. After the reaction, the precipitate was washed with deionized water until the conductivity of the dispersion reached 10 μS cm -1 the following. After concentration, Fe 3 o 4 Fe in the aqueous dispersion of nanoparticles 3 o 4 The mass fraction of nanoparticles was increased to 15%.
[0051] Weigh 0.255g of emulsifier P(E / B)-PEO, the p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Number average particle size | aaaaa | aaaaa |
| Number average particle size | aaaaa | aaaaa |
| Number average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com