Dimethyl ether water vapor reforming catalyst, and hydrogen production method thereof
A technology of steam reforming and dimethyl ether, applied in the direction of catalyst activation/preparation, chemical instruments and methods, heterogeneous catalyst chemical elements, etc., can solve the problem of high reforming temperature and achieve low CO selectivity and stability Good, overcome the effect of high reforming temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
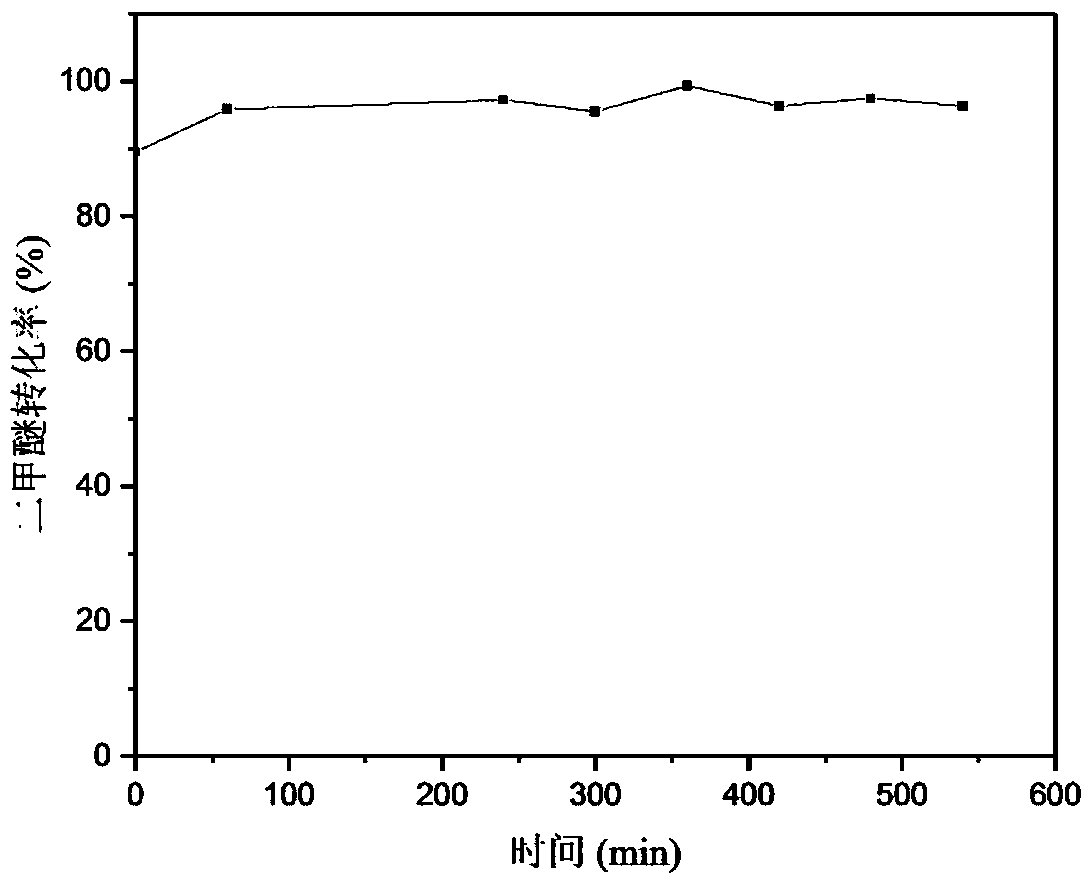
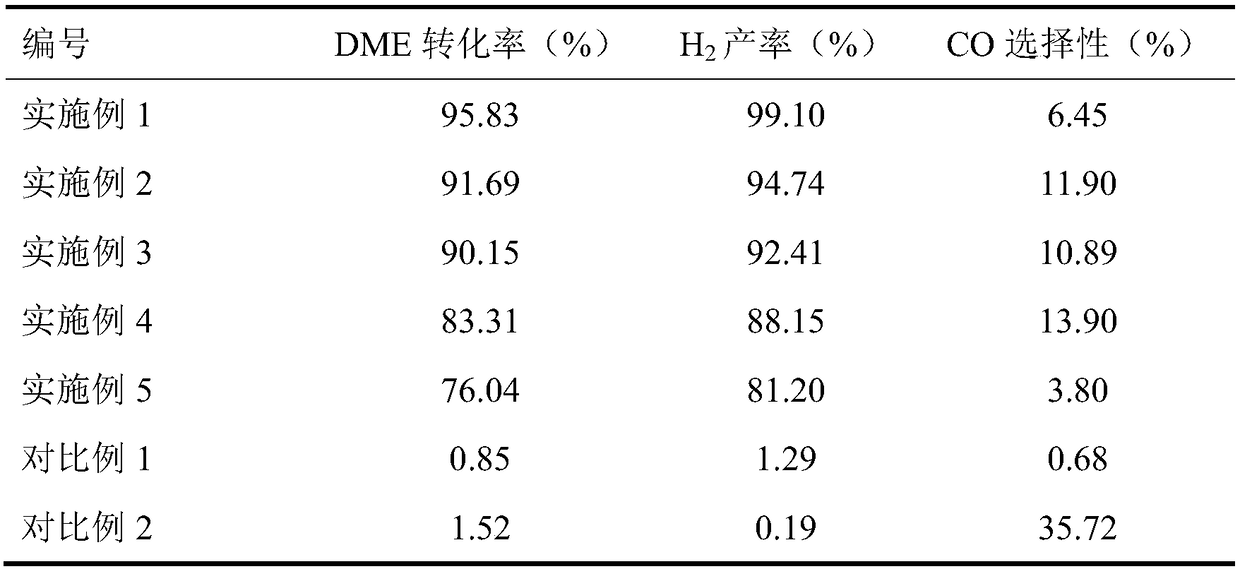
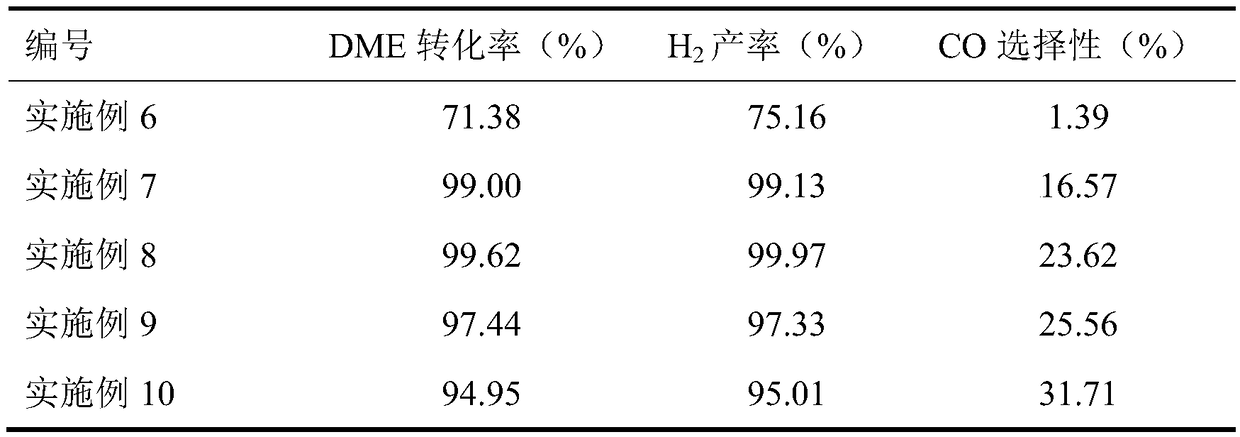
Image
Examples
Embodiment 1
[0029] (1) 0.6g zinc nitrate (Zn(NO 3 ) 2 ·6H 2 O), 5.8g zirconium nitrate (Zr(NO 3 ) 4 ·5H 2 O) dissolved in 100ml deionized water, weighed 3.06g (NH 4 ) 2 CO 3 Prepared into 100ml solution. Under the condition of stirring at 70°C, the (NH 4 ) 2 CO 3The solution was slowly added dropwise to the mixed nitrate solution to form a precipitate; continued stirring for 2 h, cooled to room temperature, filtered, and washed with deionized water for 3 times; the obtained precipitate was dried at 110 °C for 4 h under an air atmosphere, and then placed in a muffle furnace after drying. at 1℃·min -1 The temperature was raised to 500 °C and calcined at a constant temperature of 500 °C for 3 h to obtain the active component ZnO-ZrO for metal oxide solid solution reformation. 2 , where the mole fraction of ZnO in the solid solution is 13%, denoted as 13%ZnO-ZrO 2 .
[0030] (2) 13% ZnO-ZrO prepared 2 and γ-Al 2 O 3 According to the mass ratio of 2:1, the mechanical grinding ...
Embodiment 2
[0033] According to each step of embodiment 1, the difference is that step (1) is changed to 1.979g zinc nitrate (Zn(NO) 3 ) 2 ·6H 2 O), 3.875g (NH 4 ) 2 CO 3 . The mole fraction of ZnO in the solid solution is 33%, and the results are shown in Table 1.
Embodiment 3
[0035] According to each step of embodiment 1, the difference is that step (1) is changed to 4.02g zinc nitrate (Zn(NO) 3 ) 2 ·6H 2 O), 5.2g (NH 4 ) 2 CO 3 . The mole fraction of ZnO in the solid solution is 50%, and the results are shown in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com