A kind of preparation method of Limulus hemocytoprotein active peptide
A technology of protein activity and blood cells, which is applied in the field of preparation of limulus blood cell protein active peptides, can solve the problems of failure to provide experimental data on the inhibition rate of acetylcholinesterase, failure to produce acetylcholinesterase, and inability to implement specific applications. The effect of promoting application value, easy large-scale production, and less cumbersome steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
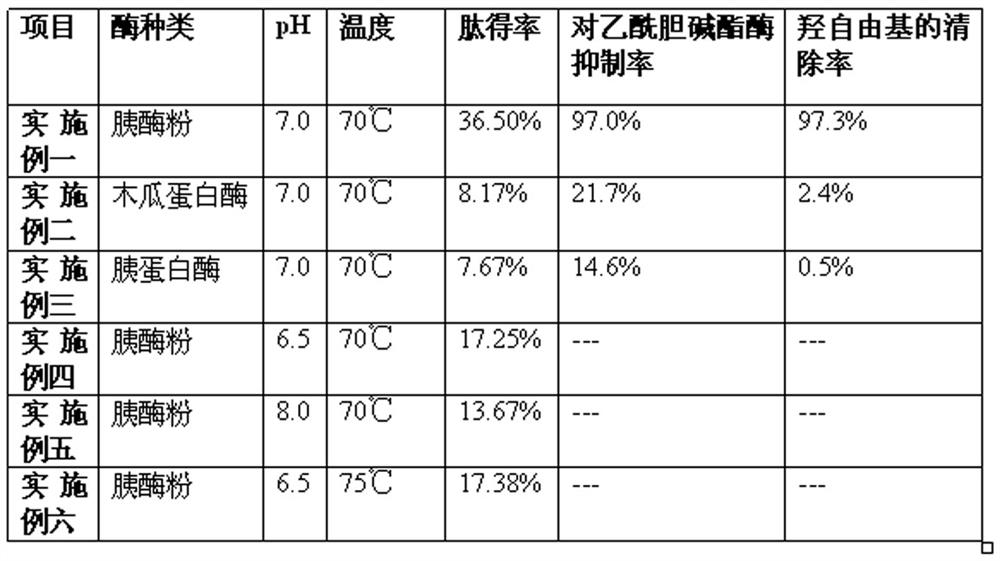
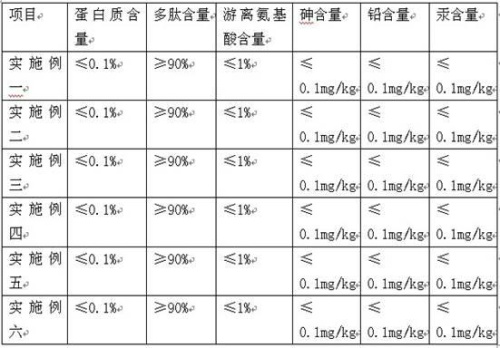
Embodiment 1
[0028] 1. Limulus blood cells were frozen at -18°C for 12 hours, then thawed at 4°C, and repeated freezing and thawing three times.
[0029] 2. Stir the Limulus blood cells after freezing and thawing three times at 4°C for 10 minutes to cause the Limulus blood cells to rupture.
[0030] 3. Keep the temperature at 4°C, centrifuge the ruptured Limulus blood cells at a low temperature of 4000 rpm for 20 minutes, collect the solid precipitate, and wash it with distilled water several times to obtain the Limulus blood cell membrane tissue.
[0031] 4. Use trypsin powder with an enzyme activity of 4000u / g to enzymolyze the Limulus blood cell membrane tissue obtained above. Enzymolysis conditions: pH 7.0, 70°C, enzyme concentration 2.2 mg / mL, substrate concentration 4.0 mg / mL, enzyme Solution 5h.
[0032] 5. Place the above enzymolysis solution in a low-temperature refrigerated centrifuge, keep the temperature at 4°C, and centrifuge at a speed of 4000rpm for 20min at a low temperatu...
Embodiment 2
[0036] 1. Limulus blood cells were frozen at -18°C for 11 hours, then thawed at 4°C, and repeated freezing and thawing three times.
[0037] 2. Stir the Limulus blood cells after freezing and thawing three times at 4°C for 10 minutes to cause the Limulus blood cells to rupture.
[0038] 3. Keep the temperature at 4°C, centrifuge the ruptured Limulus blood cells at a low temperature of 4500 rpm for 18 minutes, collect the solid precipitate, and wash it with distilled water several times to obtain the Limulus blood cell membrane tissue.
[0039] 4. Enzymolyze Limulus blood cell membrane tissue with papain, enzymolysis conditions: pH 7.0, 70°C, enzyme concentration 2.2 mg / mL, substrate concentration 4.0 mg / mL, enzymolysis 5h.
[0040] 5. Place the above enzymolysis solution in a low-temperature refrigerated centrifuge, keep the temperature at 4°C, and centrifuge at a speed of 4500rpm / min for 18min at a low temperature to obtain a supernatant.
[0041] 6. Keep the temperature at ...
Embodiment 3
[0044] 1. Limulus blood cells were frozen at -18°C, then thawed at 4°C, and repeated freezing and thawing three times.
[0045] 2. Magnetically stir the Limulus blood cells after freezing and thawing three times at 4°C for 10 minutes to cause the Limulus blood cells to rupture.
[0046] 3. Keep the temperature at 4°C, centrifuge the ruptured Limulus blood cells at a low temperature of 3800 rpm for 25 minutes, collect the solid precipitate, and wash it with distilled water several times to obtain the Limulus blood cell membrane tissue.
[0047] 4. Use trypsin to enzymolyze the Limulus blood cell membrane tissue, enzymolysis conditions: pH 7.0, 70°C, enzyme concentration 2.2 mg / mL, substrate concentration 4.0 mg / mL, enzymolysis 5h.
[0048] 5. Place the above enzymolysis solution in a low-temperature refrigerated centrifuge, keep the temperature at 4°C, and centrifuge at a speed of 3800rpm for 25min at a low temperature to obtain a supernatant.
[0049] 6. Keep the temperature at...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com