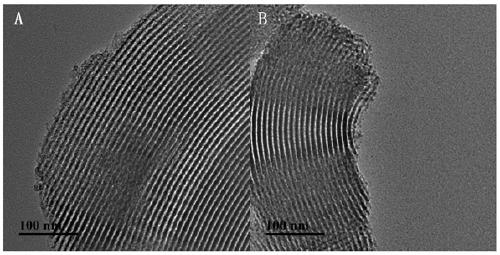
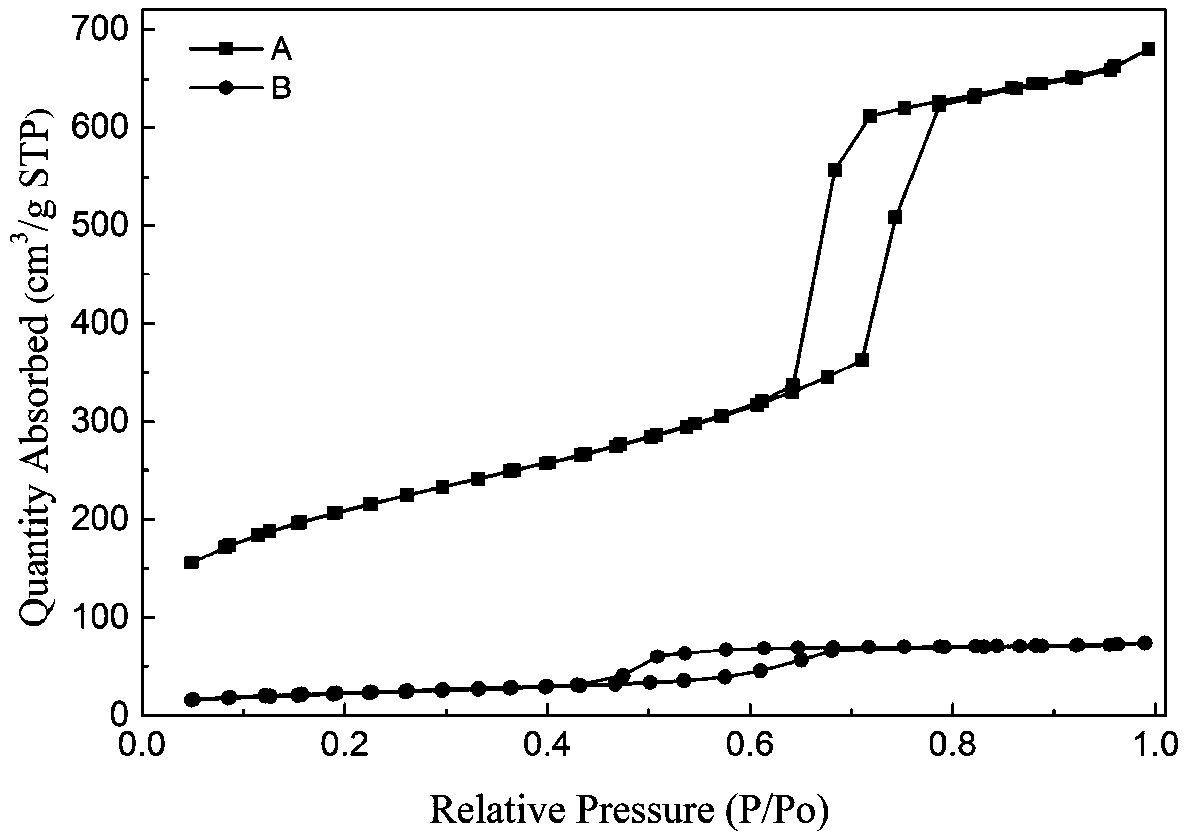
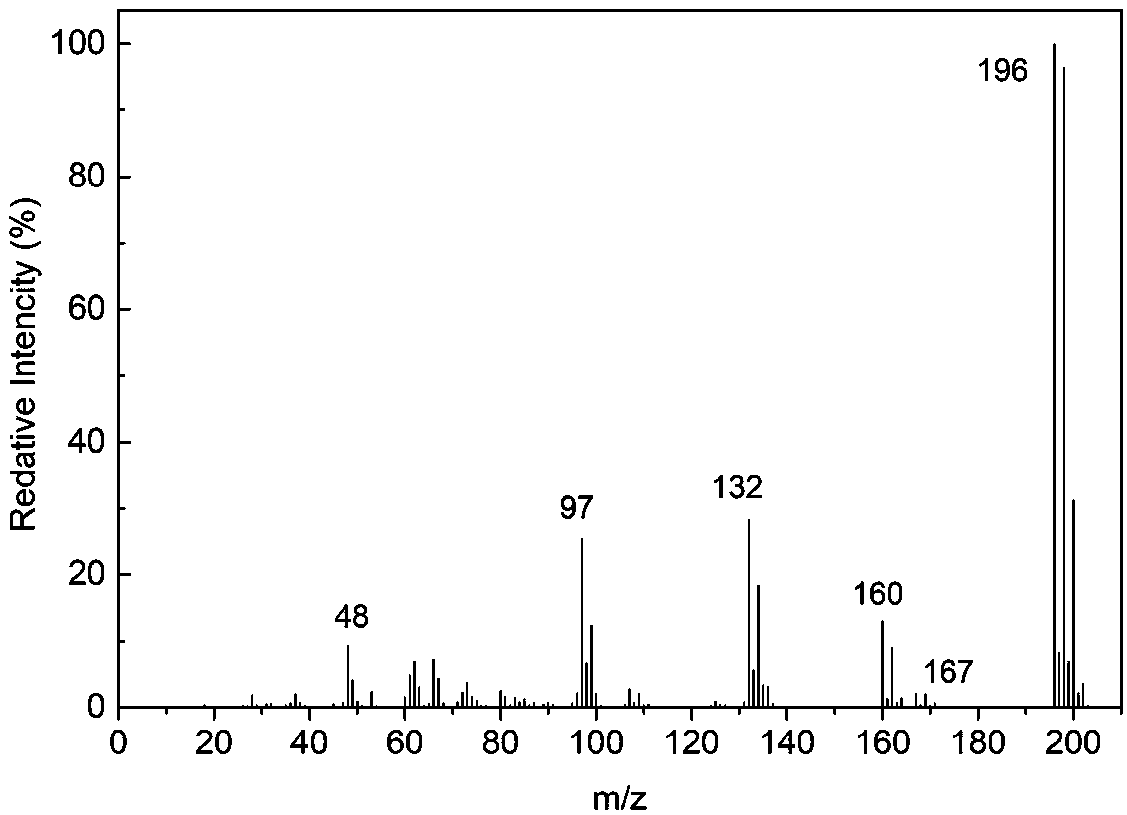
Haloamine-type macromolecular compound modified mesoporous material and application thereof
A technology of mesoporous materials and compounds, which is used in the field of macromolecular synthesis to achieve good hydrothermal stability and structural stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] A mesoporous material modified by a halogenated amine macromolecular compound, the formula ratio of which is calculated by mole fraction, and the specific preparation steps are as follows:
[0033] (1) At room temperature, react 1 part of 5,5-dimethylhydantoin with 1 part of sodium hydroxide in ethanol solution, stir magnetically for 30 minutes, and then drain the solvent to obtain 5,5-dimethylhydantoin Hydantoin sodium salt;
[0034] (2) Dissolve the sodium salt of 5,5-dimethylhydantoin obtained in step (1) in N,N-dimethylformamide, stir magnetically at 60°C for 30 minutes, and then add 1 part of 3-chloropropyl tris Ethoxysilane, reacted at 60°C for 16h, after the reaction was completed, filtered, and the solvent was removed to obtain the haloamine compound monomer;
[0035] (3) Dissolve the monomer obtained in step (2) in a mixed solution of water and ethanol (v:v=3:2), react at 80°C for 6 hours, use hydrochloric acid to adjust the pH of the reaction system to 4, and...
Embodiment 2
[0040] A mesoporous material modified by a halogenated amine macromolecular compound, the formula ratio of which is calculated by mole fraction, and the specific preparation steps are as follows:
[0041] (1) At room temperature, react 1 part of 5,5-dimethylhydantoin with 1 part of sodium hydroxide in ethanol solution, stir magnetically for 30 minutes, and then drain the solvent to obtain 5,5-dimethylhydantoin Hydantoin sodium salt;
[0042] (2) Dissolve 5,5-dimethylhydantoin sodium salt obtained in step (1) in N,N-dimethylformamide, stir magnetically at 80°C for 20 minutes, and then add 1 part of 3-chloropropyltris Ethoxysilane, react at 80°C for 12h, after the reaction, filter, remove the solvent, and obtain the haloamine compound monomer;
[0043] (3) Dissolve the monomer obtained in step (2) in a mixed solution of water and ethanol (v:v=3:2), react at 60°C for 6 hours, use hydrochloric acid to adjust the pH of the reaction system to 3, and remove the solvent after the rea...
Embodiment 3
[0051] A mesoporous material modified by a halogenated amine macromolecular compound, the formula ratio of which is calculated by mole fraction, and the specific preparation steps are as follows:
[0052] (1) At room temperature, react 1 part of 5,5-dimethylhydantoin with 1 part of sodium hydroxide in ethanol solution, stir magnetically for 30 minutes, and then drain the solvent to obtain 5,5-dimethylhydantoin Hydantoin sodium salt;
[0053] (2) Dissolve the sodium salt of 5,5-dimethylhydantoin obtained in step (1) in N,N-dimethylformamide, stir magnetically at 100°C for 10 minutes, and then add 1 part of 3-chloropropyl tris Ethoxysilane, reacted at 100°C for 8 hours, after the reaction was completed, filtered, and the solvent was removed to obtain the haloamine compound monomer;
[0054] (3) Dissolve the monomer obtained in step (2) in a mixed solution of water and ethanol (v:v=3:2), react at 40°C for 10 hours, use hydrochloric acid to adjust the pH of the reaction system to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com