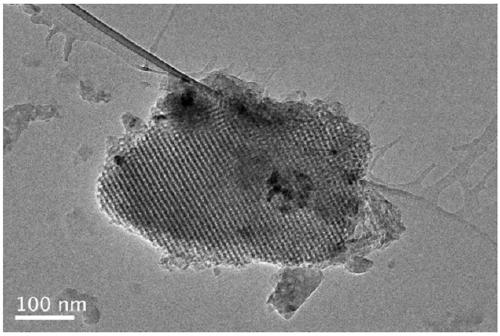
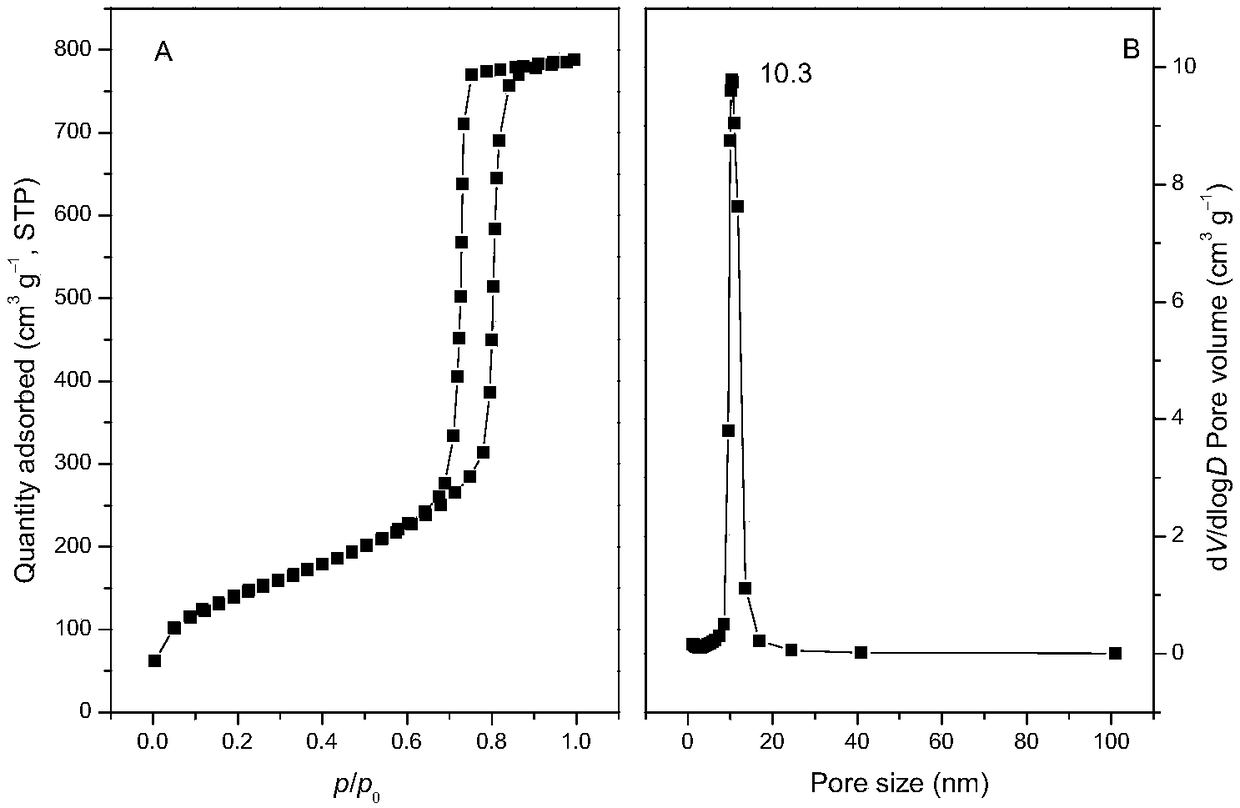
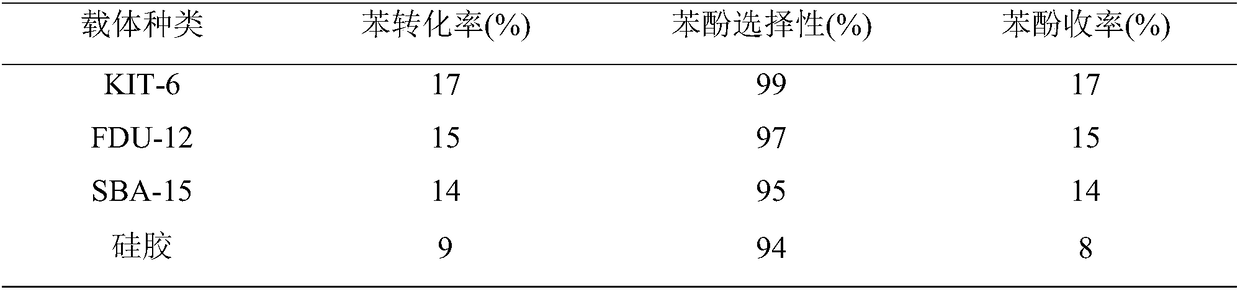
Synthesis of mesoporous catalyst for synthesizing phenol through direct hydroxylation of benzene
A catalyst and chemical synthesis technology, applied in the field of preparation of mesoporous catalysts, can solve problems such as low activity and difficult separation of products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] (1) Dissolve 10 parts by mass of mesoporous silica (KIT-6) and 5 parts by mass of APTES in toluene, and stir for 4 hours at 80°C under nitrogen protection;
[0027] (2) After the reaction, it was preliminarily washed with toluene, and then stirred and refluxed with toluene at 100°C for 2 hours, and filtered, washed and dried to obtain ammonia-functionalized mesoporous silica (KIT-6-NH 2 );
[0028] (3) 3 parts by mass of VO(acac) 2 and 5 parts by mass of KIT-6-NH 2 Dissolved in toluene, stirred and refluxed at 120°C for 20 hours, cooled, filtered, washed and dried to obtain ammonium-functionalized mesoporous silica-supported vanadyl acetylacetonate material (KIT-6-NH 2 -V);
[0029] (4) Add 0.1 parts by mass of catalyst to the acetonitrile-acetic acid solution mixed with 4.5 parts by mass of benzene and 20 parts by mass of 30 wt% hydrogen peroxide, and react at 70°C for 6h;
[0030] (5) After the reaction is completed, the catalyst is separated. The liquid phase co...
Embodiment 2
[0032] (1) Dissolve 10 parts by mass of mesoporous silica (KIT-6) and 5 parts by mass of APTMS in toluene, and stir for 2 hours at 120°C under nitrogen protection;
[0033] (2) After the reaction, it was preliminarily washed with toluene, then stirred and refluxed with toluene at 80°C for 1 h, and filtered, washed and dried to obtain ammonia-functionalized mesoporous silica (KIT-6-NH 2 );
[0034] (3) 3 parts by mass of VO(acac) 2 and 5 parts by mass of KIT-6-NH 2 Dissolved in toluene, stirred and refluxed at 100°C for 24 hours, cooled, filtered, washed and dried to obtain ammonium-functionalized mesoporous silica-supported vanadyl acetylacetonate material (KIT-6-NH 2 -V);
[0035](4) Add 0.35 parts by mass of catalyst to the acetonitrile-acetic acid solution mixed with 4.5 parts by mass of benzene and 20 parts by mass of 30 wt% hydrogen peroxide, and react at 50°C for 4h;
[0036] (5) After the reaction is completed, the catalyst is separated. The liquid phase components...
Embodiment 3
[0038] (1) Put 10 parts by mass of mesoporous silica (KIT-6) and 5 parts by mass of APTES in acetonitrile, and stir for 2 hours at 100°C under nitrogen protection;
[0039] (2) After the reaction, it was preliminarily washed with acetonitrile, and then stirred and refluxed with acetonitrile at 100°C for 2 hours, then filtered, washed and dried to obtain ammonia-functionalized mesoporous silica (KIT-6-NH 2 );
[0040] (3) 3 parts by mass of VO(acac) 2 and 5 parts by mass of KIT-6-NH 2 Dissolved in acetonitrile, stirred and refluxed at 110°C for 24h, cooled, filtered, washed and dried to obtain ammonium-functionalized mesoporous silica-supported vanadyl acetylacetonate material (KIT-6-NH 2 -V);
[0041] (4) Add 0.4 parts by mass of catalyst to the acetonitrile-acetic acid solution mixed with 4.5 parts by mass of benzene and 20 parts by mass of 30 wt% hydrogen peroxide, and react at 60°C for 6h;
[0042] (5) After the reaction is completed, the catalyst is separated. The liq...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com