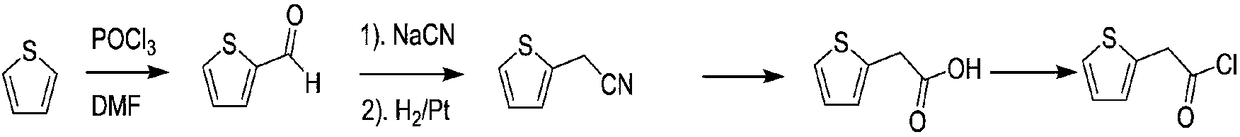
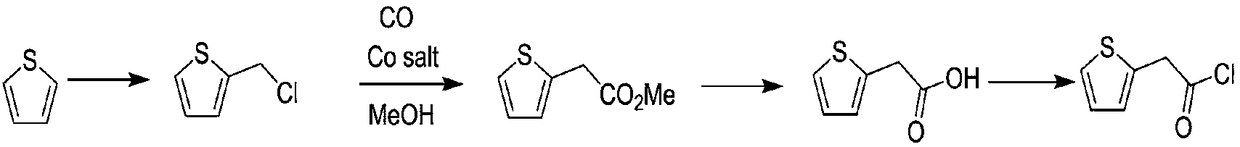
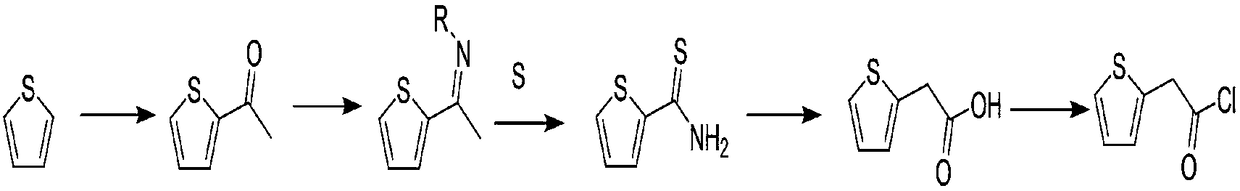
Compounding method for 2-thiopheneacetyl chloride
A technology of thiophene acetyl chloride and synthesis method, applied in directions such as organic chemistry, can solve the problems of noble iodide, unsuitable route for amplification, no simple operation, etc., and achieves the effects of easy availability of raw materials, concise route and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Preparation of ethyl 2-thiophene acetate:
[0044] Mix 20 grams of thiophene with 10 times the volume of dichloroethane, add 1.1 equivalents of ethyl glycolate and 1.1 equivalents of polyphosphoric acid, reflux for 4 hours, and cool to room temperature. The reaction solution was washed successively with cold water and saturated brine, dried and concentrated, and the obtained crude product was directly used for the next hydrolysis reaction.
[0045] Preparation of 2-thiopheneacetic acid:
[0046] Suspend the crude product obtained in the first step in 3 times the volume of ethanol, add 5 times the volume of 6N hydrochloric acid aqueous solution, reflux for 8 hours, cool to about 5°C, the product is directly precipitated, filtered, washed with water, and dried to obtain pure 2-thiopheneacetic acid. HPLC 99.2%, melting point 63-65°C.
[0047] Preparation of 2-thiopheneacetyl chloride:
[0048] Dissolve thiopheneacetic acid obtained in the second step in 10 times the vol...
Embodiment 2
[0050] Preparation of methyl 2-thiopheneacetate:
[0051] Mix 20 g of thiophene with 10 volumes of dichloroethane, add 1.1 equivalents of methyl glycolate and 1.1 equivalents of p-toluenesulfonic acid, reflux for 4 hours, and cool to room temperature. The reaction solution was washed successively with cold water and saturated brine, dried and concentrated, and the obtained crude product was directly used for the next hydrolysis reaction.
[0052] Preparation of 2-thiopheneacetic acid:
[0053] Suspend the crude product obtained in the first step in 3 times the volume of acetonitrile, add 5 times the volume of 6N hydrochloric acid aqueous solution, reflux for 8 hours, cool to about 5°C, the product is directly precipitated, filtered, washed with water, and dried to obtain pure 2-thiopheneacetic acid. HPLC 99.1%, melting point 63-65°C.
[0054] Preparation of 2-thiopheneacetyl chloride:
[0055] Dissolve thiopheneacetic acid obtained in the second step in 10 times the volume ...
Embodiment 3
[0057] Preparation of isopropyl 2-thiopheneacetate:
[0058] Mix 20 g of thiophene with 10 times the volume of dichloroethane, add 1.1 equivalents of isopropyl glycolate and 1.1 equivalents of p-toluenesulfonic acid, reflux for 4 hours, and cool to room temperature. The reaction solution was washed successively with cold water and saturated brine, dried and concentrated, and the obtained crude product was directly used for the next hydrolysis reaction.
[0059] Preparation of 2-thiopheneacetic acid:
[0060] Suspend the crude product obtained in the first step in 3 times the volume of acetonitrile, add 5 times the volume of 6N hydrochloric acid aqueous solution, reflux for 10 hours, cool to about 20°C, the product is directly precipitated, filtered, washed with water, and dried to obtain pure 2-thiopheneacetic acid. HPLC 99.1%, melting point 63-65°C.
[0061] Preparation of 2-thiopheneacetyl chloride:
[0062] Same as Example 1.
[0063] The total yield of the three steps ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com