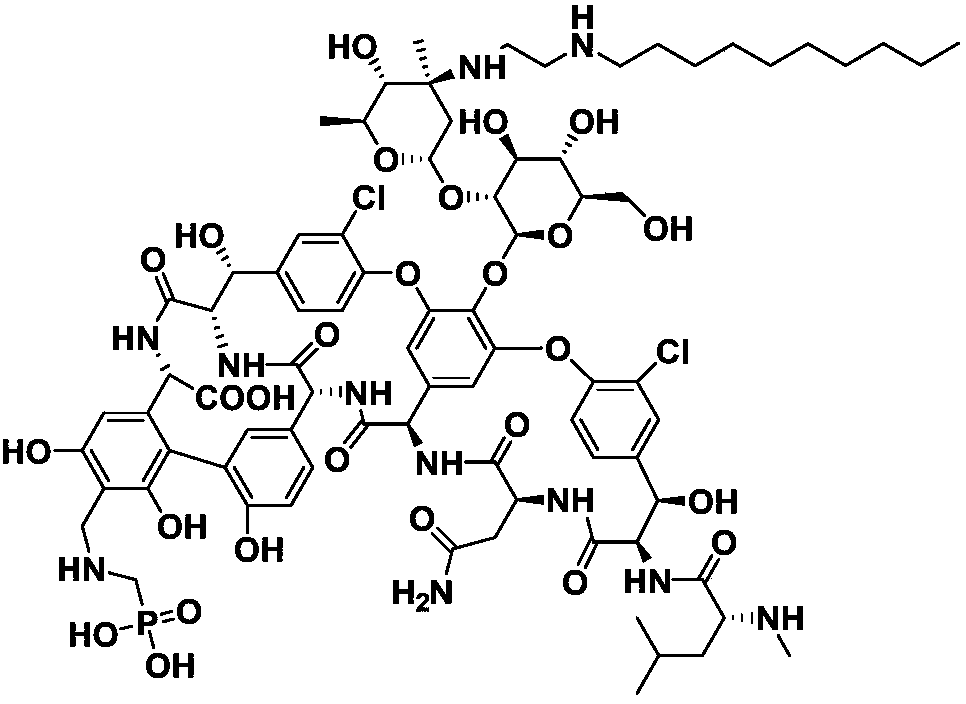
Preparation method of Telavancin
A technology of telavancin and intermediates, which is applied to the preparation of telavancin and the synthesis process of pharmaceutical intermediates, can solve the problems of high total amount of impurities and large number of telavancin impurities, and achieve high purity. , low cost, less impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
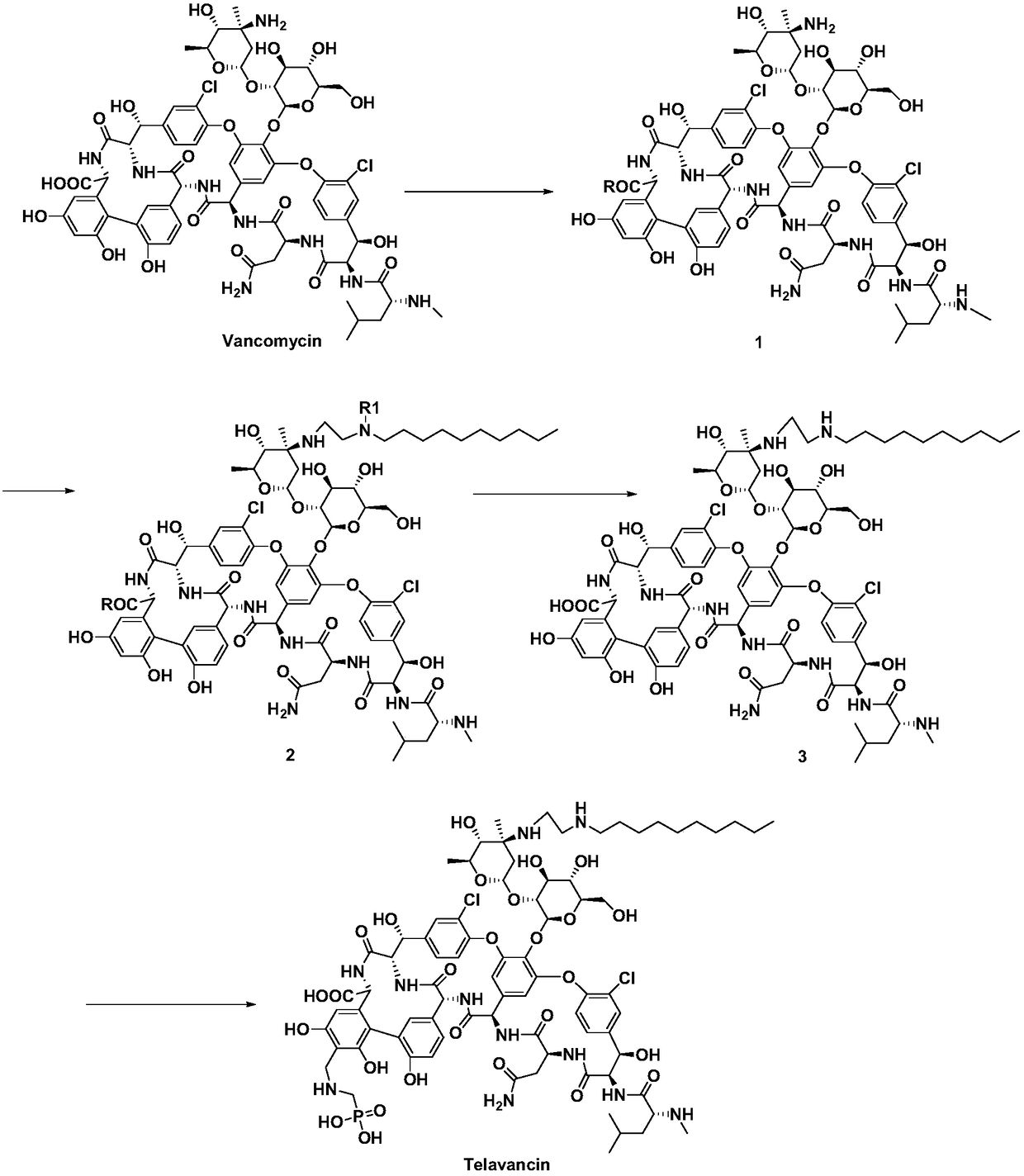
[0036] A preparation method of telavancin, comprising the steps of:
[0037] 1) Protecting the carboxyl group of vancomycin to obtain intermediate 1;
[0038] 2) Dissolve intermediate 1 and base in organic solvent A, add compound N-decyl N-R 1 -Aminoacetaldehyde undergoes aldehyde-amine condensation, and reductive amination obtains intermediate 2;
[0039] 3) removing the carboxyl protecting group and the amino protecting group of intermediate 2 to obtain intermediate 3;
[0040] 4) Intermediate 3 and aminomethyl phosphoric acid are subjected to Mannich reaction to obtain telavancin;
[0041] Its synthetic route is as follows:
[0042]
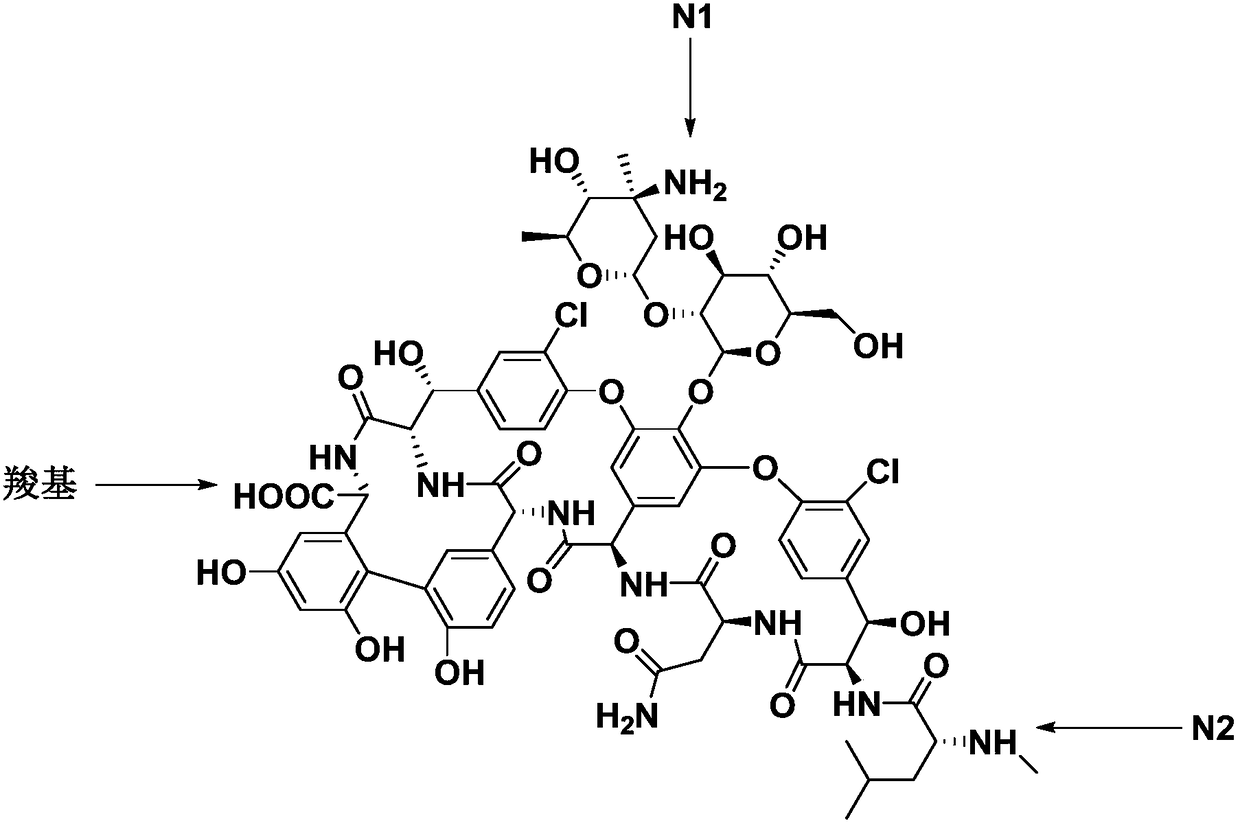
[0043] In the formula, R is a carboxyl protecting group, R 1 For the amino protecting group.
[0044] Carboxyl protection can be carried out by esterification or substitution reaction with R-OH.
[0045] The carboxyl protecting group and the amine protecting group can be commonly used protecting groups in this field, and the protectin...
Embodiment 1
[0060] Synthesis of intermediate 4:
[0061]
[0062] 1) Dissolve 320mg vancomycin in 15mL CH 3 In OH solvent, add 0.02mL concentrated sulfuric acid dropwise, and heat up to 60°C for 3h;
[0063] 2) Add 50 mL of diethyl ether, filter with suction, and wash the solid with diethyl ether; 313 mg of powder is obtained, with a yield of 97%;
[0064] Synthesis of Telavancin:
[0065]
[0066] 3) Dissolve 307mg of intermediate compound 4 in a mixed solvent of 15mL DMF and 0.25mL DIPEA, add 121mg of N-decyl N-Fmoc-aminoacetaldehyde, stir at 20-30°C for 2h, add 150mL of ether to precipitate, filter with suction, and use for filter cake Wash and filter with ether;
[0067] 4) Dissolve the filter cake directly in 10mL DMF, slowly add 25mg NaBH in batches at 20-30°C 3 CN and 5mL CH 3 OH, 0.1mL TFA, react at 20-30°C for 2h, add 100mL ether and suction filter, wash the filter cake with 50mL ether and water successively, and dry to obtain 250mg of white solid;
[0068] 5) Dissolv...
Embodiment 2
[0070] Synthesis of Intermediate 6:
[0071]
[0072] 1) Dissolve 300mg vancomycin in 15mL (CH 3 ) 2 In CHOH solvent, add 0.02mL of concentrated sulfuric acid dropwise, and heat up to 60°C for 3h;
[0073] 2) Add 50 mL of diethyl ether, filter with suction, and wash the solid with diethyl ether; obtain 303 mg of powder with a yield of 98%;
[0074]
[0075] 3) Dissolve 250mg of intermediate compound 6 in a mixed solvent of 15mL DMF and 0.25mL DIPEA, add 106mg of N-decyl N-Fmoc-aminoacetaldehyde, stir at 20-30°C for 2h, add 150mL of ether to precipitate, filter with suction, and use for filter cake Wash and filter with ether;
[0076] 4) Dissolve the filter cake directly in 10mL DMF, slowly add 14mg NaBH in batches at 20-30°C 3 CN and 5mL CH 3 OH, 0.1mL TFA, react at 20-30°C for 2h, add 100mL ether and filter with suction, wash the filter cake with 50mL ether and water successively, and dry to obtain 246mg of white solid;
[0077] 5) Dissolve 148mg of aminomethylpho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com