Method for determining contents of chlorpheniramine maleate and acetaminophen in Ankahuangmin tablet
A technology for acetaminophen and chlorpheniramine acid, which is applied in the field of drug testing, can solve problems such as the lack of control of the content of chlorpheniramine maleate, and achieve improved quality controllability, good separation, and repeatability Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Embodiment 1: the content determination of chlorpheniramine maleate in Ankahuangmin tablets
[0056] In the present embodiment, the reference substance solutions are all chlorpheniramine maleate reference substance solutions; both chlorpheniramine maleate and acetaminophen double negative control solutions are chlorpheniramine maleate and acetaminophen double negative control solutions. Negative control solution ①; need testing solution is need testing solution ①.
[0057] System suitability test:
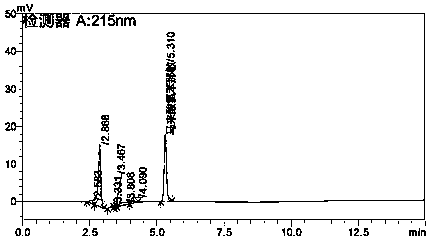
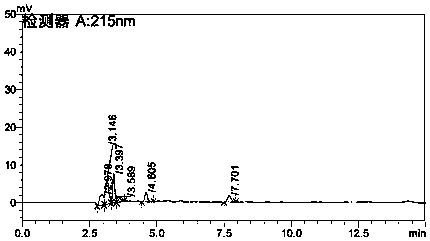
[0058] Inject the chlorpheniramine maleate reference substance solution, the chlorpheniramine maleate and acetaminophen double negative control solution and the test solution into the liquid chromatograph, record the chromatogram, and the blank control solution is at the position of the reference substance Without interfering peaks, the number of theoretical plates of the chlorpheniramine maleate sample solution under the above conditions is 13915, the degree of separation ...
Embodiment 2
[0083] Embodiment 2: Determination of the content of acetaminophen in Ankahuangmin Tablets
[0084] In this embodiment, the reference substance solution is all paracetamol reference substance solution; both chlorpheniramine maleate and paracetamol double negative control solution are both chlorpheniramine maleate and paracetamol double negative control solution ②; the test solution is the test solution ②.
[0085] System suitability test:
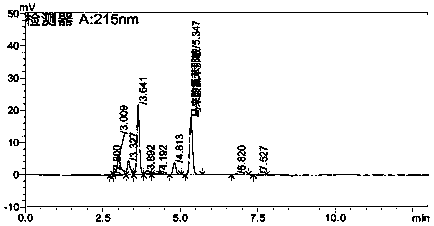
[0086] Inject paracetamol reference substance solution, double negative control solution and need testing solution into liquid chromatograph, record the chromatogram, blank control solution has no interference peak at the position of reference substance, under the above conditions paracetamol sample solution The number of theoretical plates is 14547, the resolution is 37.6, and the tailing factor is 1.16. The reference values are in line with the 2010 Pharmacopoeia regulations. Specific as Figure 5 , Figure 6 , Figure 7 .
[008...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| separation | aaaaa | aaaaa |
| separation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com