A two-layer or multi-layer polymer electrolyte and a battery
An electrolyte and polymer technology, applied in the field of double-layer polymer electrolyte, can solve the problems of reducing battery energy density and cycle performance, difficulty reaching 300Wh/kg, and violating solid-state batteries, etc., to assist lithium ion transport and avoid reaction loss , Promote the effect of lithium salt dissolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] The double-layer electrolyte structure provided by this embodiment is as figure 1 shown. In this embodiment, there is a double-layer electrolyte composed of two layers of polymer electrolytes between the positive electrode and the negative electrode, wherein the positive electrode is lithium cobaltate (LiCoO 2 , LCO), the negative electrode is lithium metal. The first layer of polymer electrolyte is a solid electrolyte that is electrochemically stable to high voltage, and it is in contact with the positive electrode. The polymer electrolyte used is poly(N-methyl-malonic amide (Poly(N-methyl-malonic amide, PMA); the second polymer electrolyte layer is a solid electrolyte that is electrochemically stable at low voltage, which is in contact with the negative electrode, and the polymer electrolyte used is polyethylene oxide (PEO) containing lithium salt.
[0053] figure 1 A schematic diagram of the double-layer electrolyte structure.
[0054] In conventional liquid Li-i...
Embodiment 2
[0059] Performance Test of Double Layer Solid Electrolyte Membrane
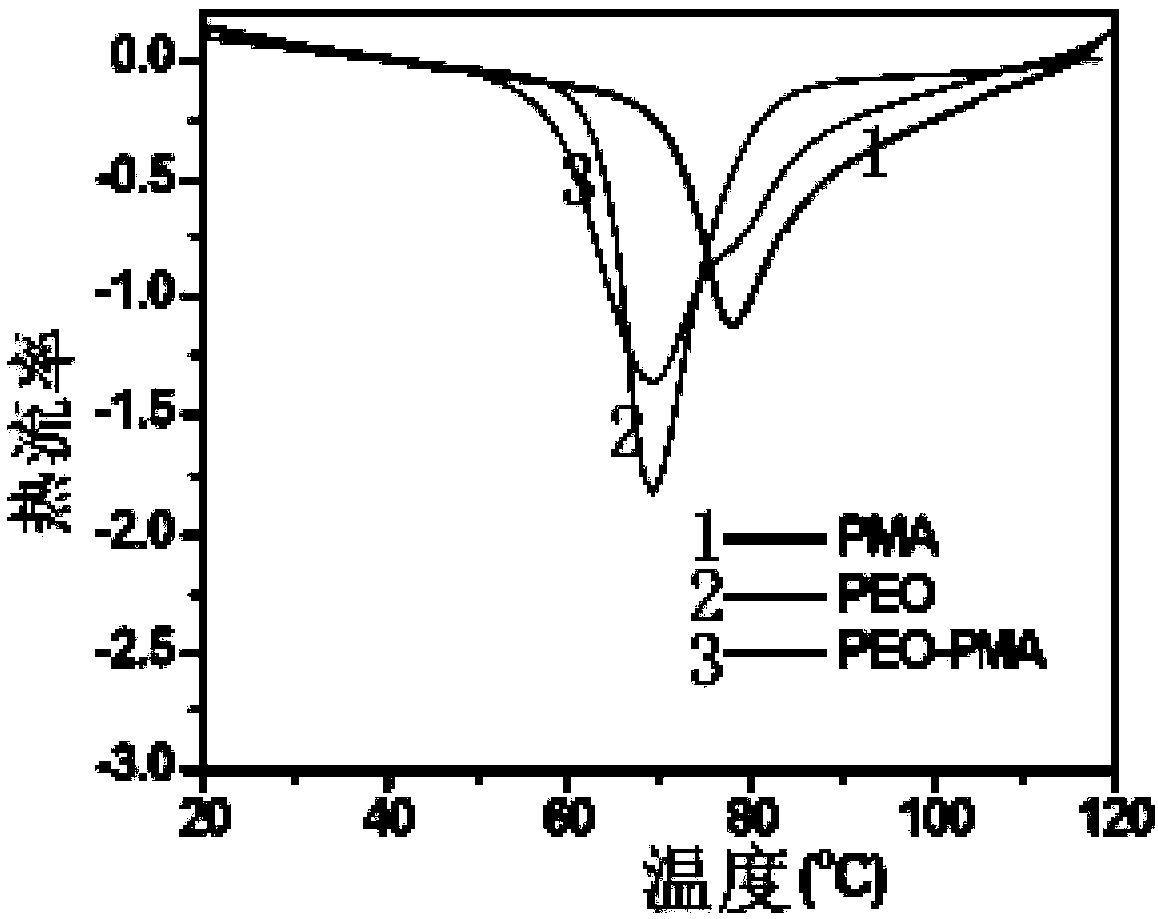
[0060] Differential scanning calorimeter (DSC) was used to test DLPSE, and the two endothermic peaks obtained in the range of 65-85 °C corresponded to the melting points of the PEO layer and the PMA layer (such as figure 2 shown).
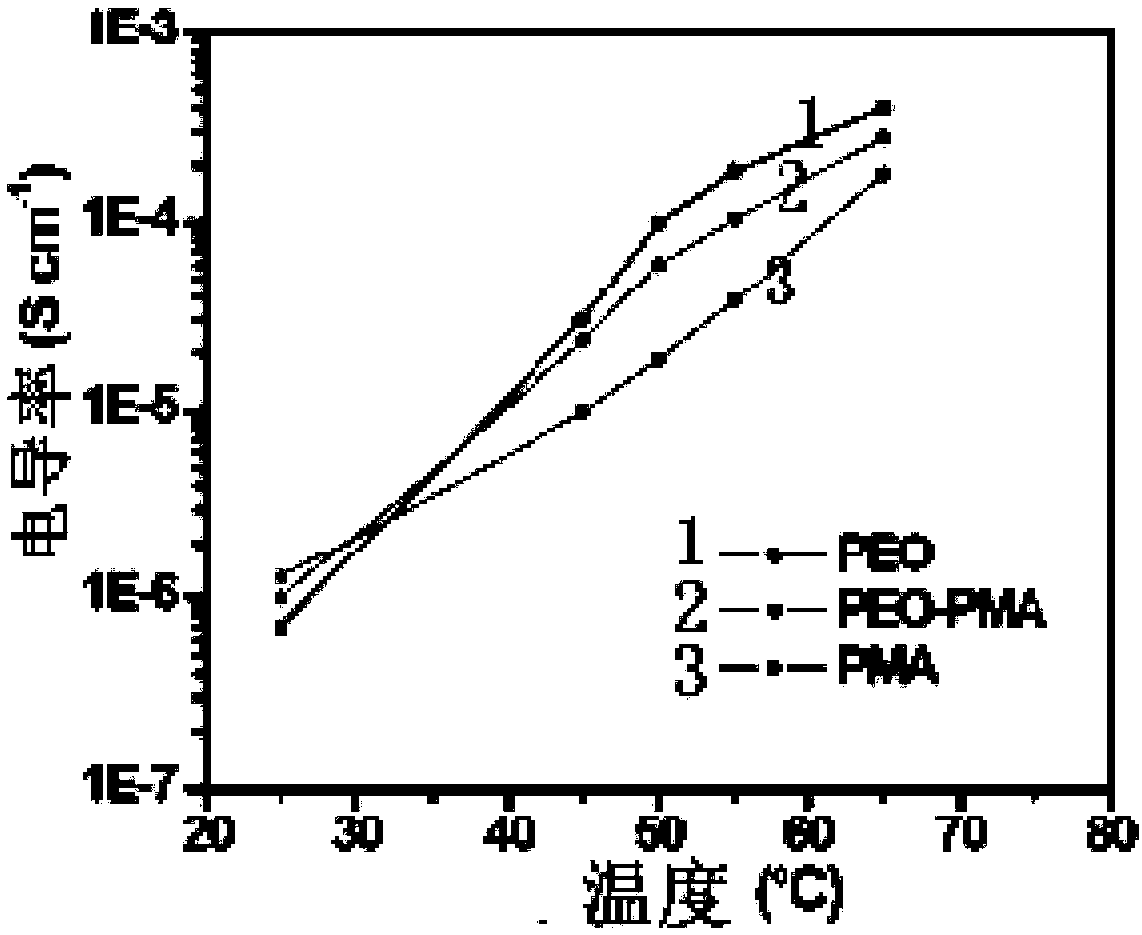
[0061] figure 2 Show the DSC curve graph of PEO, PMA and PEO / PMA three kinds of polymers, image 3 The ion conductivities of PEO, PMA and PEO / PMA three polymers at different temperatures are shown.
[0062] image 3 The ionic conductivities of PEO-Li thin films, PMA-Li thin films and DLPSE at different temperatures (25-65°C) are shown. We can notice that the ionic conductivity of DLPSE in the high temperature region (50–65°C) is between that of PEO-Li and PMA-Li, which indicates that due to the good adhesion between the polymers, PEO-Li and PMA-Li The interface resistance is sufficiently low. Such as image 3 As shown, the conductivity of the dry PMA-Li film at 65 °C is a...
Embodiment 3
[0066] Performance testing of all-solid-state lithium-ion batteries
[0067] To evaluate the electrochemical performance of double-layer electrolytes for practical batteries, we assembled the Li / DLPSE / LiCoO 2 Battery.
[0068] Figure 8 Li / DLPSE / LiCoO 2 The voltage curve of the battery in the first 5 weeks and 100 weeks, the voltage test range is 2.5 ~ 4.2V vs Li / Li + , with a current density of 0.2C (100μA cm -2 ), we can observe that LiCoO 2 The characteristic charge-discharge curves; moreover, the discharge capacity gradually increased in the first 5 weeks due to the electrode infiltration.
[0069] Figure 9 It shows that when the current density is 0.2C, the capacity can be maintained at 108.5mAh g after 100 charge-discharge cycles –1 , up to the highest discharge capacity (119mAh g –1 ), demonstrating the electrochemical stability in charge-discharge cycles.
[0070] Figure 10 It shows that when the current density is 0.1C, 0.2C and 0.5C, the battery capacity ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
| Ionic conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com