Stepwise removing method of phosphate and sulfate radicals in iron phosphate wastewater
A technology of iron phosphate and phosphate radicals, which is applied in chemical instruments and methods, preparation/separation of ammonia, water pollutants, etc. It can solve the problems that phosphate radicals and sulfate radicals cannot be removed and recovered step by step, and achieve a high degree of resource utilization Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
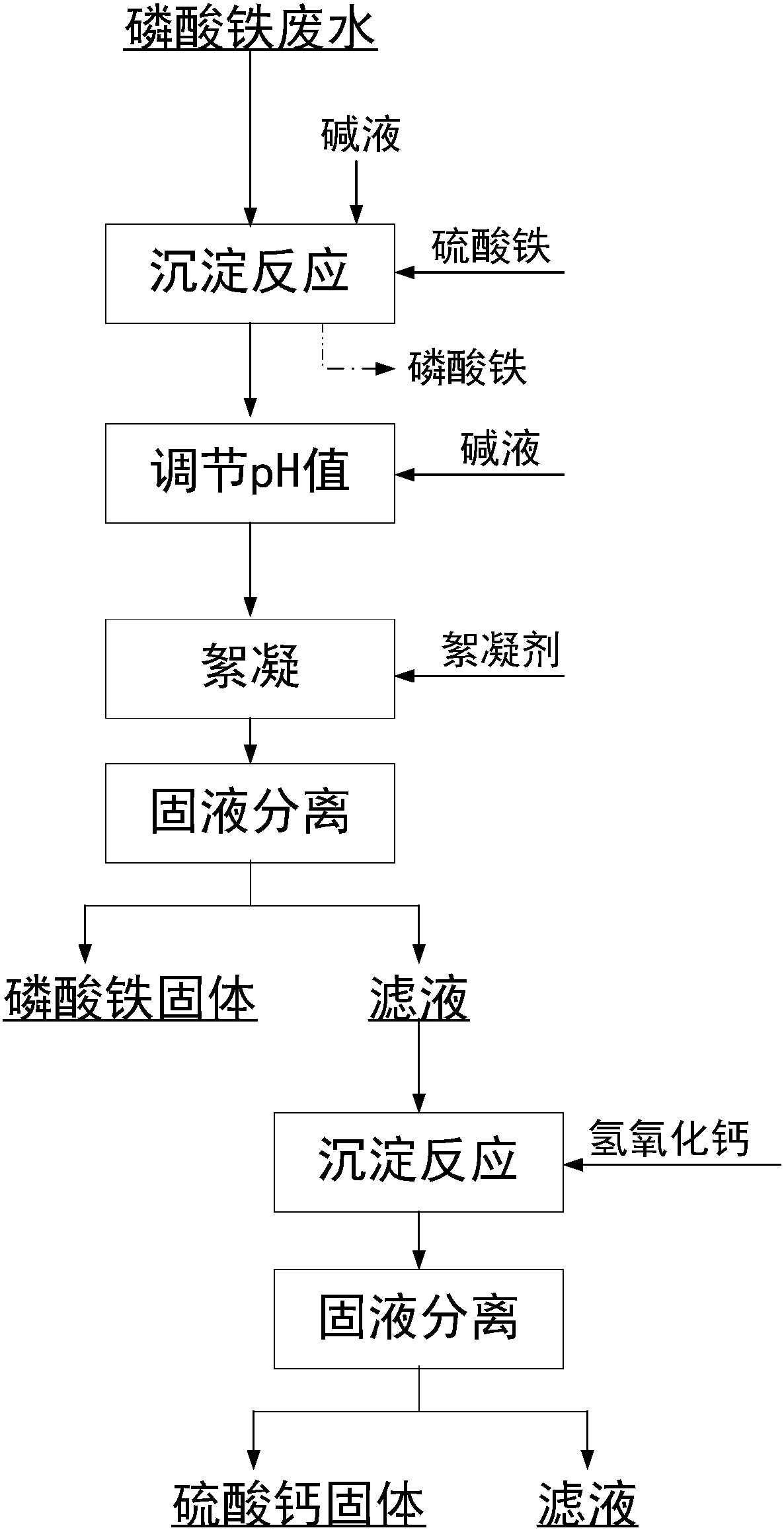
[0065] The step-by-step removal of phosphate and sulfate in iron phosphate wastewater, the specific steps are as follows:
[0066] 1) Get ferric phosphate production wastewater, test the concentration of phosphate and sulfate radicals therein, and add lye to adjust the pH=6-9 of wastewater;
[0067] 2) According to the concentration of the tested phosphate radical, add ferric sulfate solid with the number of moles of iron ions being 0.5 times of the phosphate ion, and stir for 30 minutes;
[0068] 3) Add lye again to adjust pH=3-3.5, and stir for 2 minutes;
[0069] 4) Add 5% PFC, the volume ratio to ferric phosphate wastewater is 1:2000, stir for 10 minutes, settle for 30 minutes, filter to obtain solid and filtrate containing ferric phosphate, and analyze the sulfate concentration and phosphate concentration in the filtrate ;
[0070] 5) Determination of the sulfate concentration in the filtrate in step 4), and adding calcium hydroxide solid with the molar ratio of calcium...
Embodiment 2
[0078] The step-by-step removal method of phosphate and sulfate in iron phosphate wastewater, the specific steps are as follows:
[0079] 1) Get ferric phosphate production wastewater, test the concentration of phosphate and sulfate radicals therein, and add lye to adjust the pH=6-9 of wastewater;
[0080] 2) According to the concentration of the tested phosphate radical, add ferric sulfate solid with the number of moles of iron ions being 2 times of the phosphate ion, and stir for 2 hours;
[0081] 3) Add lye again to adjust pH=7-8, and stir for 2 minutes;
[0082] 4) Add 3% PAS and stir for 2 minutes, the volume ratio to the ferric phosphate wastewater is 1:500, let the precipitation stand for 5 minutes, filter to obtain a solid and filtrate containing ferric phosphate, and analyze the sulfate concentration and phosphate concentration in the filtrate;
[0083] 5) measure step 4) the sulfate radical concentration in the filtrate, and be 1.2:1 to add calcium hydroxide solid w...
Embodiment 3
[0091] The step-by-step removal method of phosphate and sulfate in iron phosphate wastewater, the specific steps are as follows:
[0092] 1) Get ferric phosphate production wastewater, test the concentration of phosphate and sulfate radicals therein, and add lye to adjust the pH=6-9 of wastewater;
[0093] 2) According to the concentration of the tested phosphate radical, add ferric sulfate solid with the number of moles of iron ions being 1.2 times of the phosphate ion, and stir for 30 minutes;
[0094] 3) Add lye again to adjust pH=3.5-4.5, and stir for 2 minutes;
[0095] 4) Add 0.5% PFS and stir for 5 minutes, the volume ratio to the ferric phosphate wastewater is 1:1500, let the precipitate stand for 30 min, filter to obtain a solid containing ferric phosphate and filtrate, and analyze the sulfate concentration and phosphate concentration in the filtrate;
[0096] 5) Determination of the sulfate concentration in the filtrate in step 4), and adding calcium hydroxide solid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com