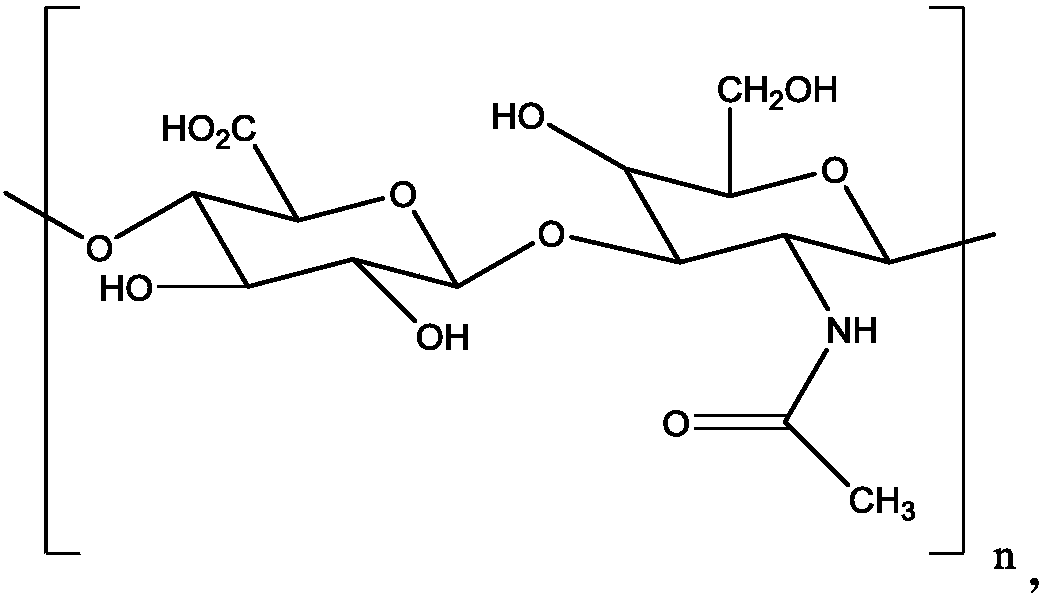
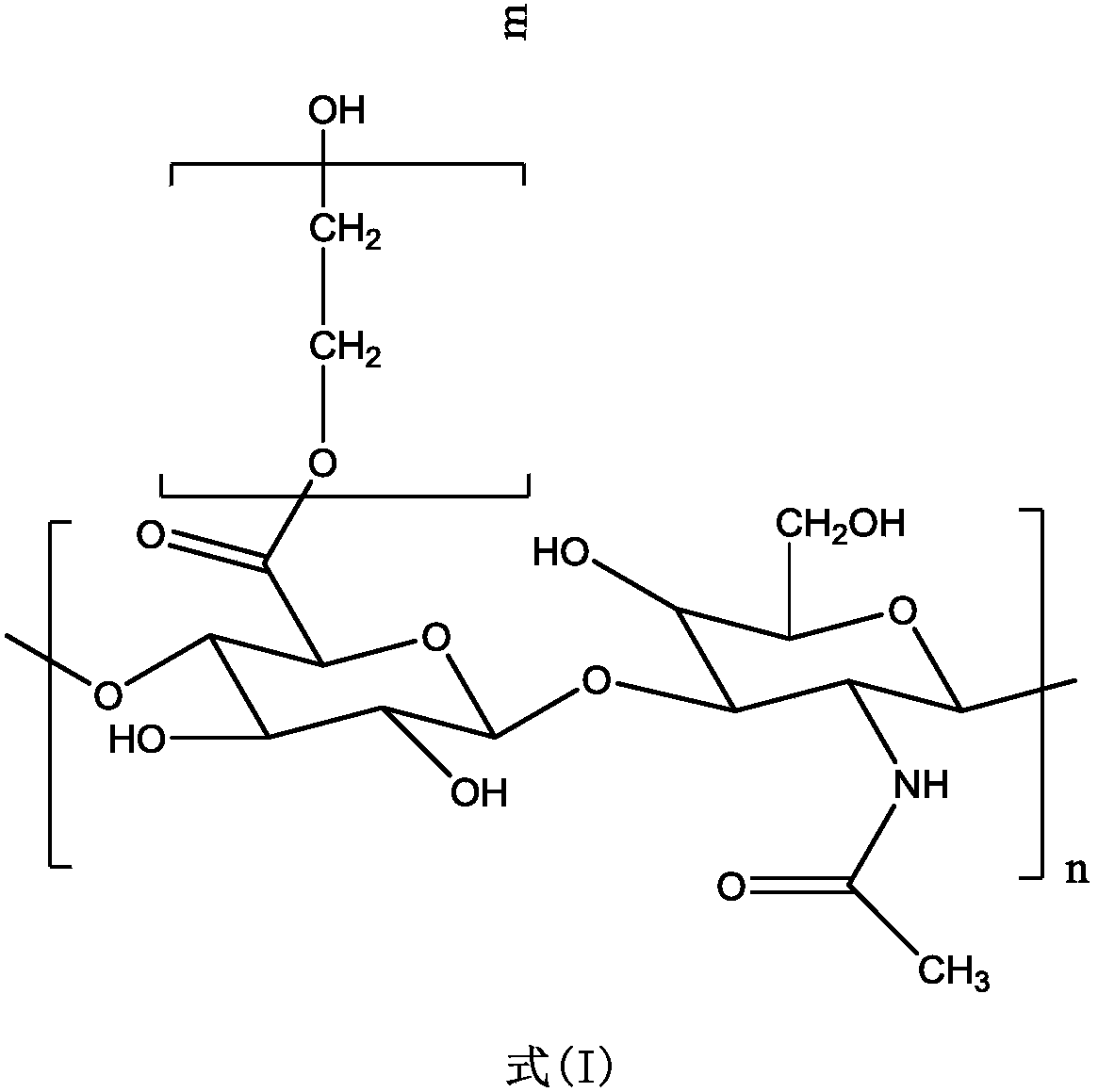
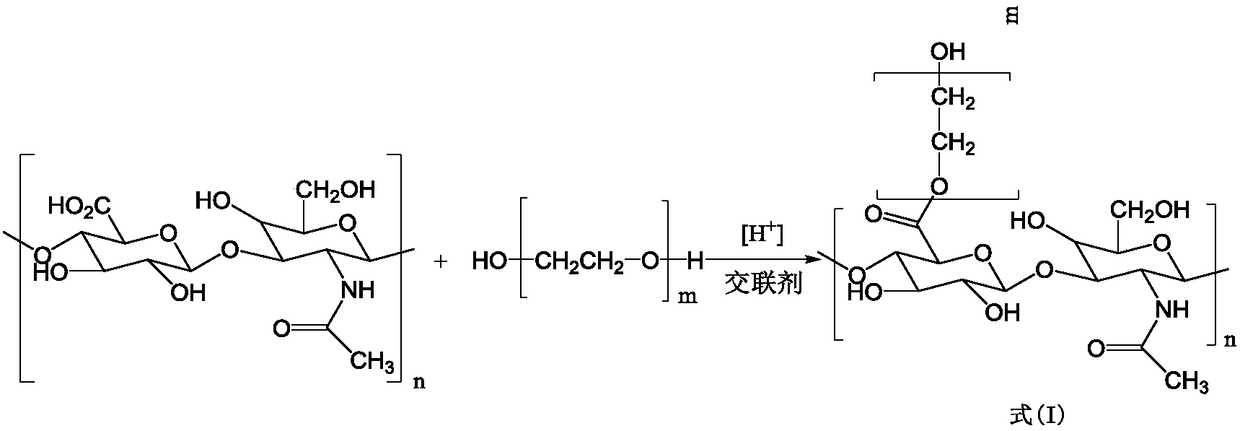
Modified hyaluronic acid injectable filling material and preparation method thereof
A filling material, hyaluronic acid technology, applied in pharmaceutical formulations, pharmaceutical sciences, prostheses, etc., can solve the problems of non-disclosure, and achieve the effects of good stability, good biocompatibility, and stable metabolism
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Step (1) Preparation of cross-linked hyaluronic acid gel:
[0040] a. Weigh hyaluronic acid / polyethylene glycol / HOBc / EDC (hyaluronic acid / polyethylene glycol / HOBt / EDC=1g / 0.5g / 0.1 / 0.1,w / w) according to a certain ratio, and dissolve them in 50mL deionized Stir well in water to make it evenly mixed;
[0041] b. Add NaH to the reaction system 2 PO 4 -NaHPO 4 , control the pH value of the reaction system=5,;
[0042] c. Control the reaction temperature to 60-80°C and stir thoroughly for 12 hours;
[0043] d. Dialyzing the reaction product to obtain a cross-linked hyaluronic acid gel.
[0044] Step (2): Weigh free transparent hyaluronic acid gel aqueous solution (molecular weight: 50KDa) according to the proportion (free transparent hyaluronic acid gel: cross-linked hyaluronic acid gel = 0.8g / 1g, w / w), and add to the solution prepared in step (1). Preparation of the prepared cross-linked hyaluronic acid gel, and fully mixing and stirring at room temperature;
[0045] S...
Embodiment 2
[0048] Step (1) Preparation of cross-linked hyaluronic acid gel:
[0049] a. Weigh hyaluronic acid / polyethylene glycol / HOBc / EDC (hyaluronic acid / polyethylene glycol / HOBt / EDC=1g / 1g / 0.1 / 0.1,w / w) according to a certain ratio, and dissolve them in 50mL deionized water Stir well to make it evenly mixed;
[0050] b. Add NaH to the reaction system 2 PO4 -NaHPO 4 , control the pH value of the reaction system=5,;
[0051] c. Control the reaction temperature to 60-80°C and stir thoroughly for 12 hours;
[0052] d. Dialyzing the reaction product to obtain a cross-linked hyaluronic acid gel.
[0053] Step (2): Weigh the free transparent hyaluronic acid gel aqueous solution (molecular weight is 50KDa) according to the proportion (free transparent hyaluronic acid gel: cross-linked hyaluronic acid gel = 0.5g / 1g, w / w), add to step (1) Preparation of the prepared cross-linked hyaluronic acid gel, and fully mixing and stirring at room temperature;
[0054] Step (3): Weigh water-soluble vi...
Embodiment 3
[0057] Step (1) Preparation of cross-linked hyaluronic acid gel:
[0058] a. Weigh hyaluronic acid / polyethylene glycol / HOBc / EDC (hyaluronic acid / polyethylene glycol / HOBt / EDC=0.5g / 1 / 0.1 / 0.1,w / w) according to a certain ratio, and dissolve them in 50mL deionized Stir well in water to make it evenly mixed; b. Add NaH to the reaction system 2 PO 4 -NaHPO 4 , control the pH value of the reaction system=5;
[0059] c. Control the reaction temperature to 60-80°C and stir thoroughly for 12 hours;
[0060] d. Dialyzing the reaction product to obtain a cross-linked hyaluronic acid gel.
[0061] Step (2): Weigh the free transparent hyaluronic acid gel aqueous solution (molecular weight is 50KDa) according to the proportion (free transparent hyaluronic acid gel: cross-linked hyaluronic acid gel = 0.5g / 1g, w / w), add to step (1) Preparation of the prepared cross-linked hyaluronic acid gel, and fully mixing and stirring at room temperature;
[0062] Step (3): Weigh water-soluble vitamin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| storage modulus | aaaaa | aaaaa |
| storage modulus | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com