A method for preparing a solid ferric oxide-loading activated carbon Fenton's reagent
A Fenton reagent, activated carbon technology, applied in chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, oxidized water/sewage treatment, etc., can solve the problem of unstable chemical properties of ferrous ions, ferrous ions problems such as loss, activity dependence, etc., to achieve the effect of promoting treatment effect, good catalytic degradation, and decreasing dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
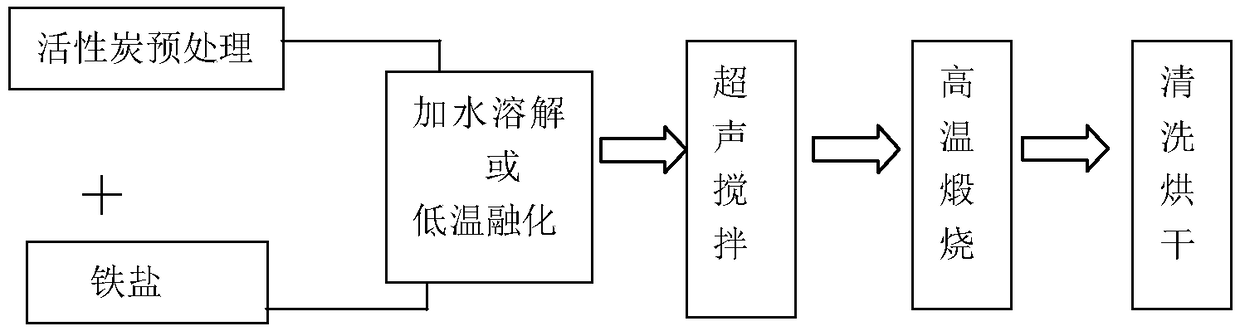
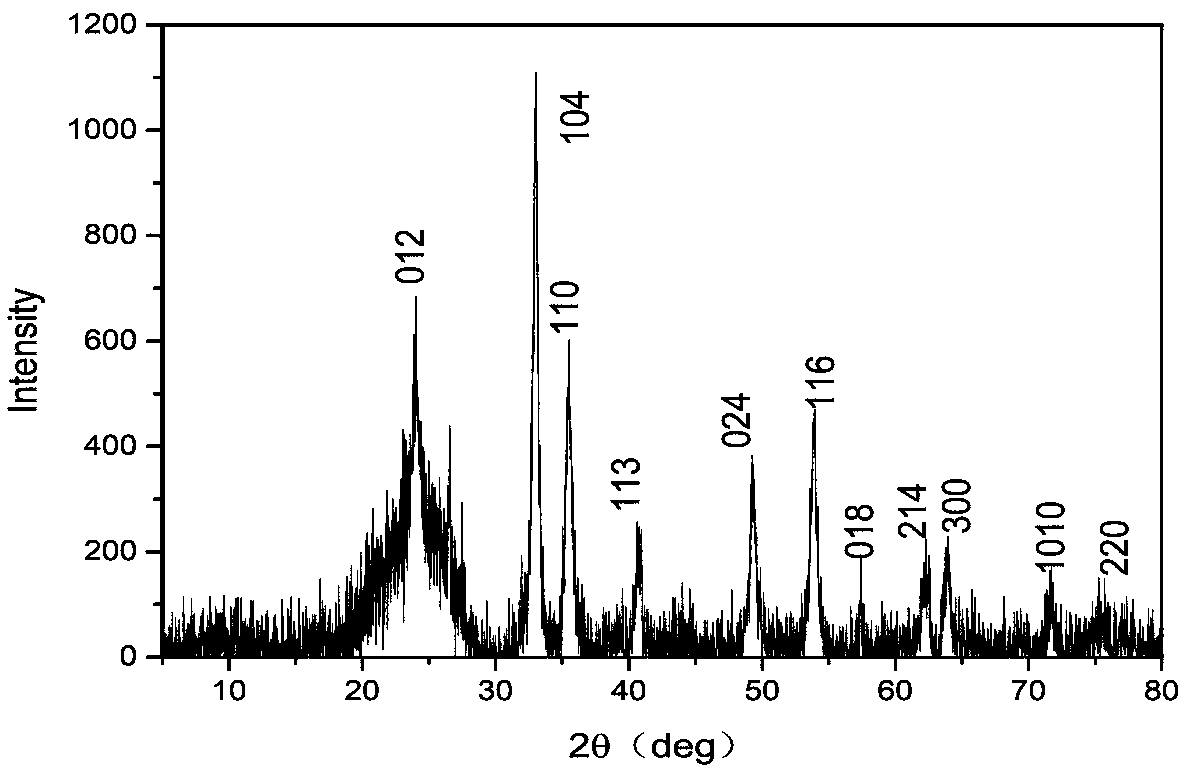
[0020] The activated carbon powder was boiled in 2mol / L nitric acid solution for 2 hours, then soaked for 24 hours, washed with deionized water until the pH value was neutral, and dried for later use. Mix ferric nitrate nonahydrate and acid-treated activated carbon powder at a mass ratio of 4:1, gradually add a small amount of deionized water until the mixture is muddy, shake the muddy mixture in a constant temperature oscillator for 6 hours, and place it in a blast Dry the water in a drying oven at 60°C, transfer to a muffle furnace for calcination at 250°C for 3 hours, and naturally cool to obtain dark brown solid particles, which are ground to fine powder. The sample that embodiment 1 is made is dropped into in the dye wastewater of 100mg / L, and wastewater pH value is neutral, and the dosage of Fenton's reagent is 0.5g / L, and the dosage of hydrogen peroxide is 30mmol / L, after After 20 minutes of reaction, the color of the wastewater completely faded and turned into a transp...
Embodiment 2
[0023] The activated carbon powder was boiled in 2mol / L nitric acid solution for 2 hours, then soaked for 24 hours, washed with deionized water until the pH value was neutral, and dried for later use. Mix ferric chloride hexahydrate and acid-treated activated carbon powder at a mass ratio of 5:1, heat in a blast drying oven at 60°C for half an hour, ferric chloride hexahydrate melts into a liquid state, stir for 3 hours, and then sonicate After 15 minutes, it was transferred to a muffle furnace and calcined at 200°C for 2 hours. After natural cooling, dark brown solid particles were obtained, which were ground into fine powder. The sample that embodiment 2 is made is dropped in the dye waste water of 100mg / l, and the dosage of Fenton's reagent is 0.5g / L, and potassium persulfate dosage is 20mmol / L, through 20 minutes reaction, the color of wastewater Fade off completely to a clear solution.
Embodiment 3
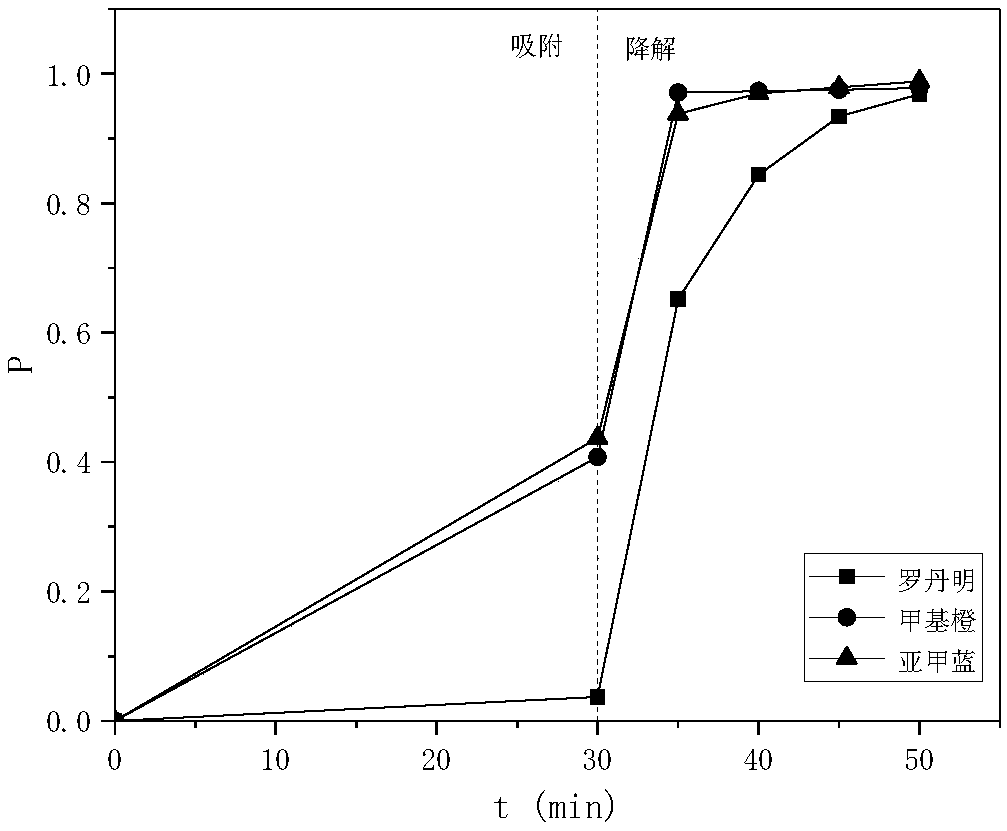
[0025] The Fenton's reagent obtained in Example 2 is recycled, and the test conditions are as follows: the rhodamine dye wastewater concentration is 100mg / l, the dosage of Fenton's reagent is 0.5g / L, and the dosage of hydrogen peroxide is 30mmol / L L, reaction time 40 minutes. The decolorization rates of the first to fourth uses are 100%, 100%, 92% and 68% respectively; the dissolution rates of iron ions are 2.3, 1.7, 1.2 and 0.6mg / L respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com