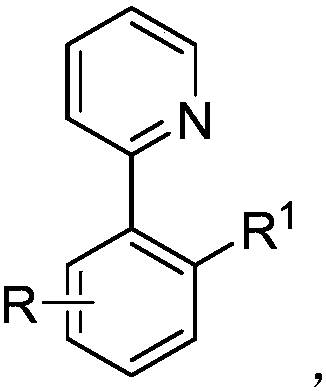
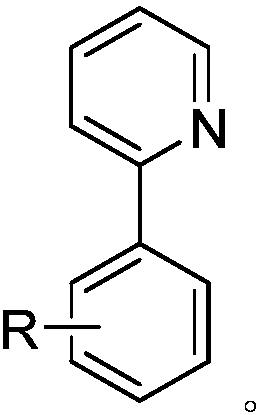
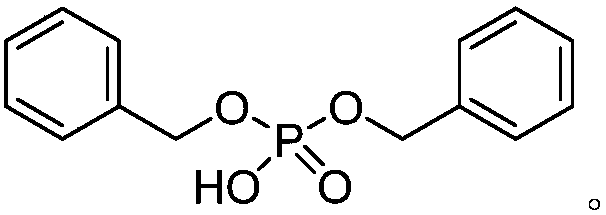
Alkyl modified aryl pyridine compound and preparation method thereof
A technology for arylpyridines and compounds, which is applied in the field of alkyl-modified arylpyridines and their preparation, can solve the problems of high price, side reactions, and low utilization of raw materials, and achieves simple operation, mild reaction conditions, and reduced The effect of chemical waste generation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Palladium-Catalyzed Reaction of Arylpyridines with Iodobutane
[0031] Palladium catalyzed arylpyridine and iodobutane reaction synthetic alkyl substituted arylpyridine arene compound, comprises the following steps: in the Schlenk reaction tube of 35ml, add stirring bar successively, 0.45mg Pd(OAc) 2 (10mol%), the corresponding arylpyridine (0.2mmol), 91.0uL iodobutane (0.8mmol), 44.5mg (BnO) 2 PO 2 H (80mol%), 165.4mgAg 2 CO 3 (3.0equiv) and 1.0mL of t-AmylOH:CH 3 CN (9:1) organic solvent, then seal the reaction tube with a matching polytetrafluoroethylene stopper, and place it in a magnetic stirrer at 60°C for 12 hours. At the end of the reaction, the reaction tube was removed from the heating device and cooled to room temperature. The reaction solution was diluted with ethyl acetate and filtered through diatomaceous earth. The filtrate obtained after washing several times with ethyl acetate was concentrated with a rotary evaporator to obtain the crude product Pu...
Embodiment 2
[0164] Palladium-Catalyzed Reaction of 2-Phenylpyridine with Iodoalkane
[0165] Into a 35ml Schlenk reaction tube, add a stirring bar, 0.45mg Pd(OAc) 2 (10mol%), the corresponding 28.6uL 2-phenylpyridine (0.2mmol), iodoalkane (0.8mmol), 44.5mg (BnO) 2 PO 2 H (80mol%), 165.4mgAg 2 CO 3(3.0equiv) and 1.0mL of t-AmylOH:CH 3 CN (9:1) organic solvent, then seal the reaction tube with a matching polytetrafluoroethylene stopper, and place it in a magnetic stirrer at 60°C for 12 hours. At the end of the reaction, the reaction tube was removed from the heating device and cooled to room temperature. The reaction solution was diluted with ethyl acetate and filtered through diatomaceous earth. The filtrate obtained after washing several times with ethyl acetate was concentrated with a rotary evaporator to obtain the crude product Purify and isolate the corresponding butyl compound through silica gel plate, weigh to determine the yield, and use NMR and HRMS for qualitative detection....
Embodiment 3
[0216] 2-Phenylpyridine, iodobutane, dibenzyl phosphate, silver carbonate and Pd(TFA) 2 Mix at a molar ratio of 1:4:0.8:3:0.01, dissolve in the solvent tert-amyl alcohol, then seal the reaction tube with a matching polytetrafluoroethylene stopper, and place it in a magnetic stirrer at 80°C for 12 hours. At the end of the reaction, the reaction tube was removed from the heating device and cooled to room temperature. The reaction solution was diluted with ethyl acetate and filtered through diatomaceous earth. The filtrate obtained after washing several times with ethyl acetate was concentrated with a rotary evaporator to obtain the crude product The corresponding butyl compound was obtained by purification and isolation on a silica gel plate, and the yield was calculated by weighing. The final product is:
[0217]
[0218] The yields were 76% and 12%, respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com