Lenvatinibsolid dispersion and preparation method thereof and preparation
A technology of solid dispersion and lenvatinib, which is applied in the direction of active ingredients of heterocyclic compounds, drug combination, powder delivery, etc., can solve the problem of low bioavailability of lenvatinib, and improve bioavailability and production cost Low, increase the effect of dissolution rate and dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
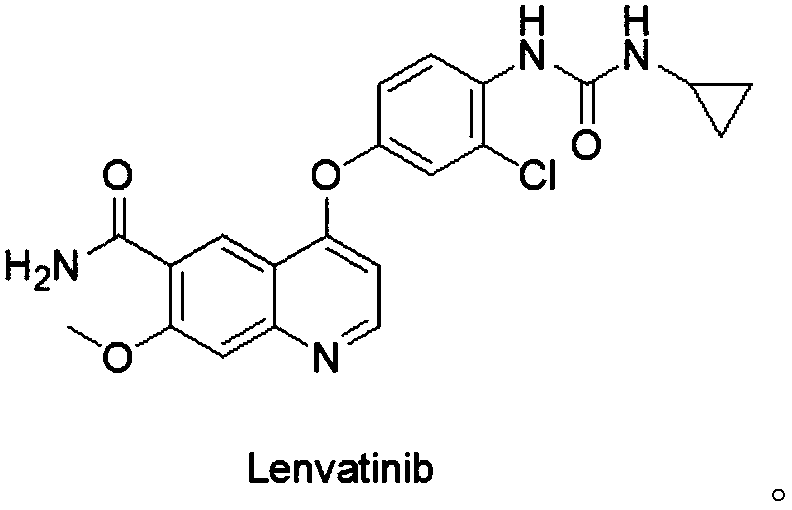
[0035] A solid dispersion of lenvatinib, which comprises lenvatinib and a water-soluble carrier material; the mass ratio of lenvatinib and the water-soluble carrier material is 10:1-1:10.
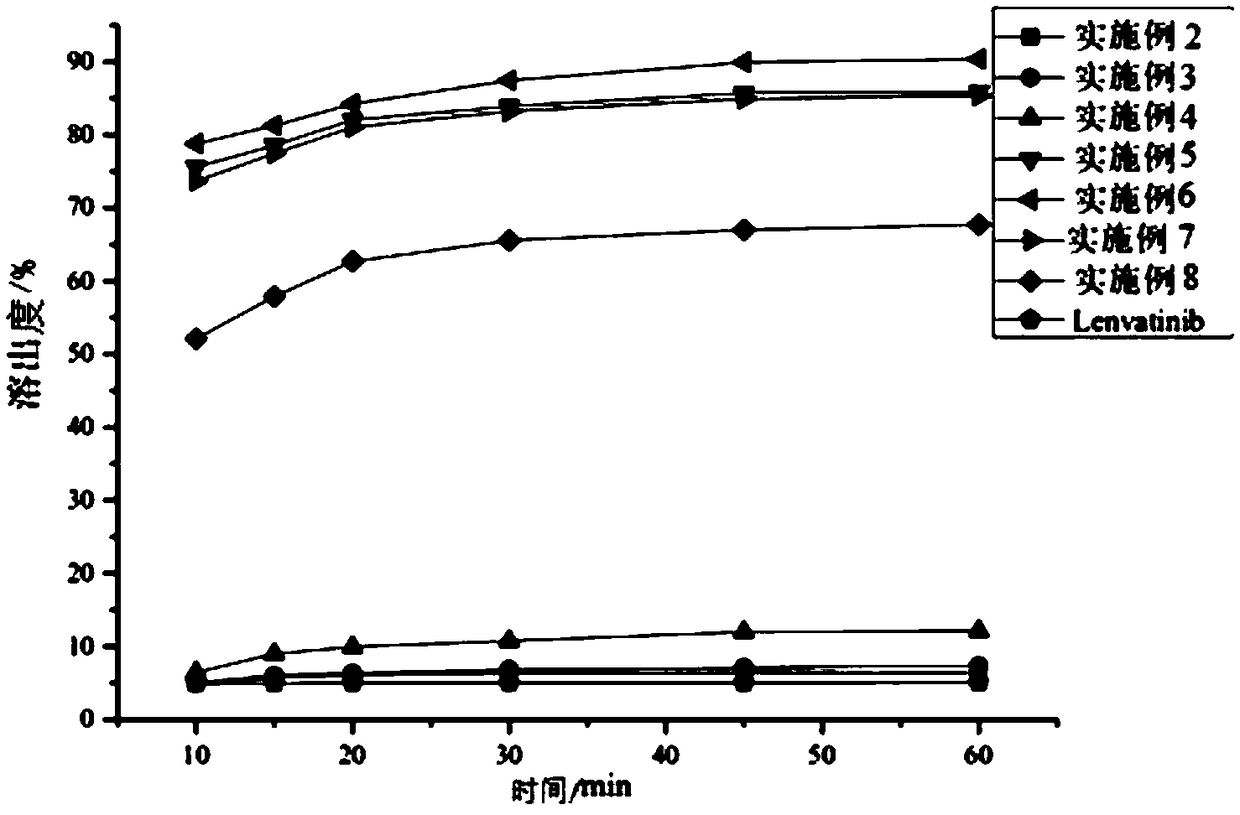
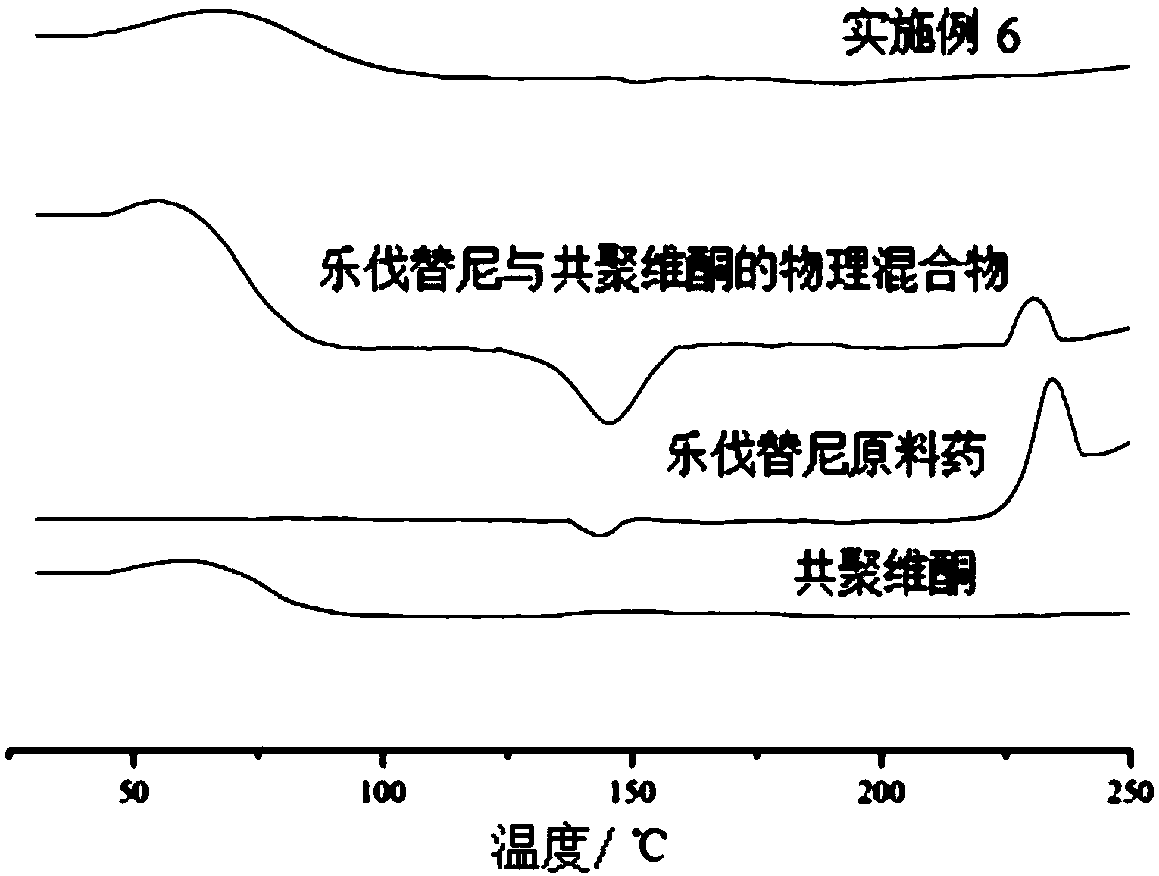
[0036] The lenvatinib solid dispersion of this embodiment uses water-soluble carrier materials as the carrier of lenvatinib, which can convert needle-like crystal lenvatinib into amorphous lenvatinib, and is highly dispersed in water-soluble The carrier material greatly improves the dissolution rate and dissolution rate of lenvatinib, thereby improving the bioavailability of lenvatinib.
[0037] In this embodiment, the water-soluble carrier material is preferably polyethylene glycol 3000, polyethylene glycol 4000, polyethylene glycol 6000, polyethylene glycol 8000, povidone K15, povidone K25, povidone K30 , povidone K60, povidone K90, copovidone, stearic acid, sodium dodecylbenzene sulfonate, quaternary ammonium compound, lecithin, amino acid type, betaine type, fatty acid glyceride, fatty ...
Embodiment 2
[0047] A solid dispersion of lenvatinib, which is specifically prepared through the following steps:
[0048] 1) Add 60mg of lenvatinib and 40mg of copovidone into 20ml of methanol, heat up to 55°C and stir for 30min to obtain reaction solution A;
[0049] 2) distilling off the methanol in the reaction solution A to obtain the initial lenvatinib solid dispersion;
[0050] 3) The initial lenvatinib solid dispersion is vacuum-dried, wherein the drying temperature is 30°C, the drying time is 12h, and ground and passed through an 80-mesh sieve to obtain a white powdery lenvatinib solid that meets the particle size requirements Dispersions.
Embodiment 3
[0052] A solid dispersion of lenvatinib, which is specifically prepared through the following steps:
[0053] 1) Add 50mg of lenvatinib and 50mg of copovidone into 20ml of methanol, heat up to 55°C and stir for 30min to obtain reaction solution A;
[0054] 2) distilling off the methanol in the reaction solution A to obtain the initial lenvatinib solid dispersion;
[0055] 3) The initial lenvatinib solid dispersion is vacuum-dried, wherein the drying temperature is 30°C, the drying time is 12h, and ground and passed through an 80-mesh sieve to obtain a white powdery lenvatinib solid that meets the particle size requirements Dispersions.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com