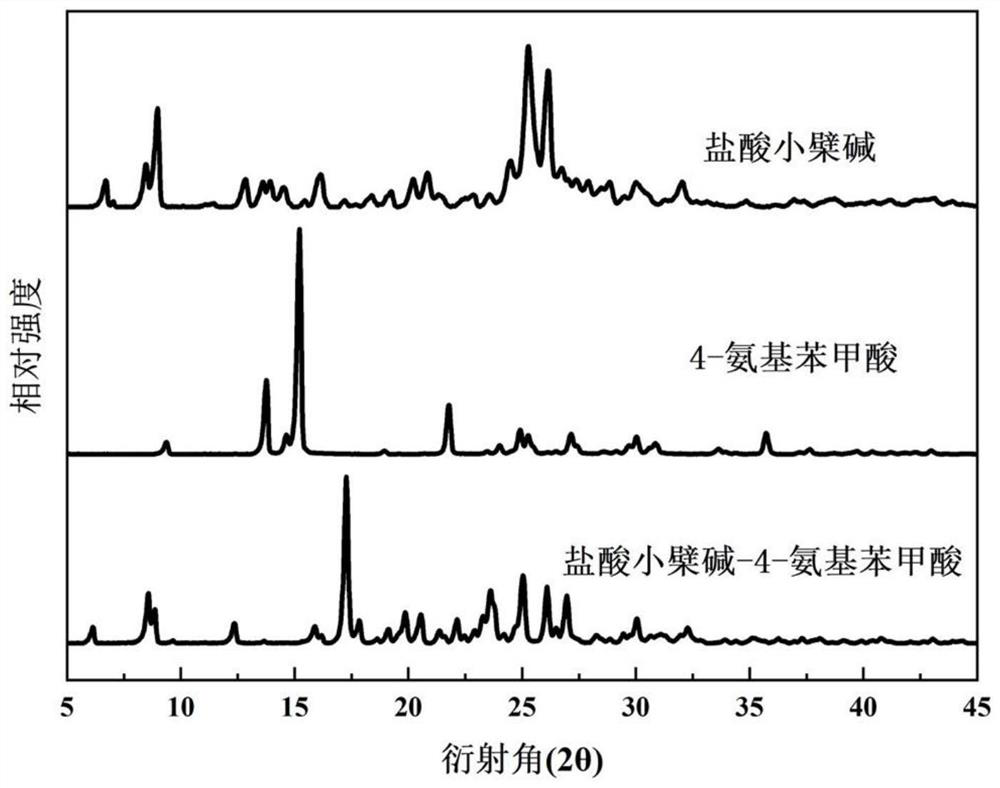
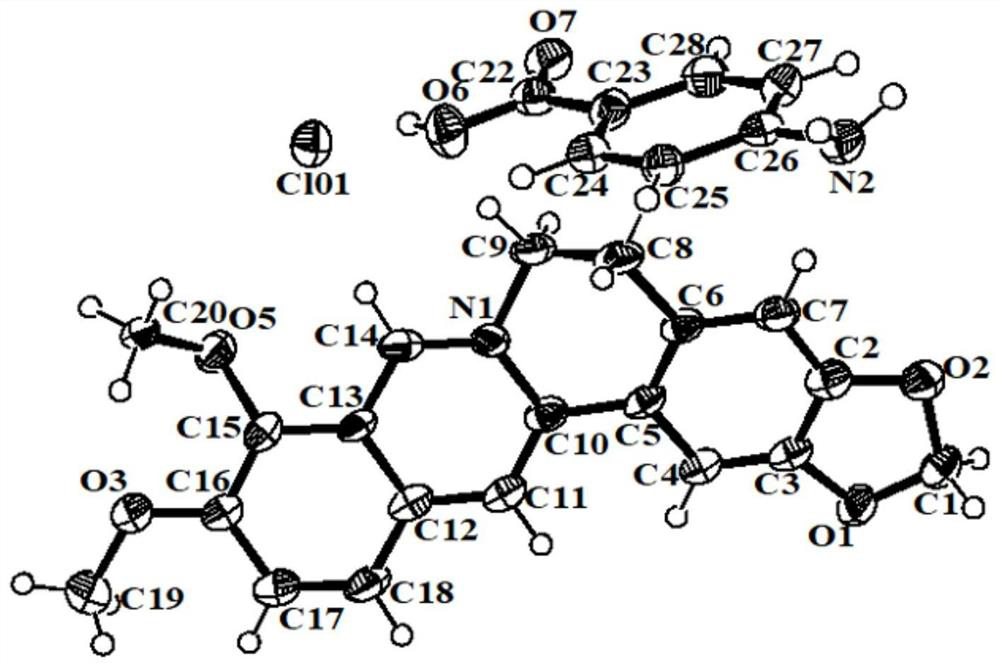
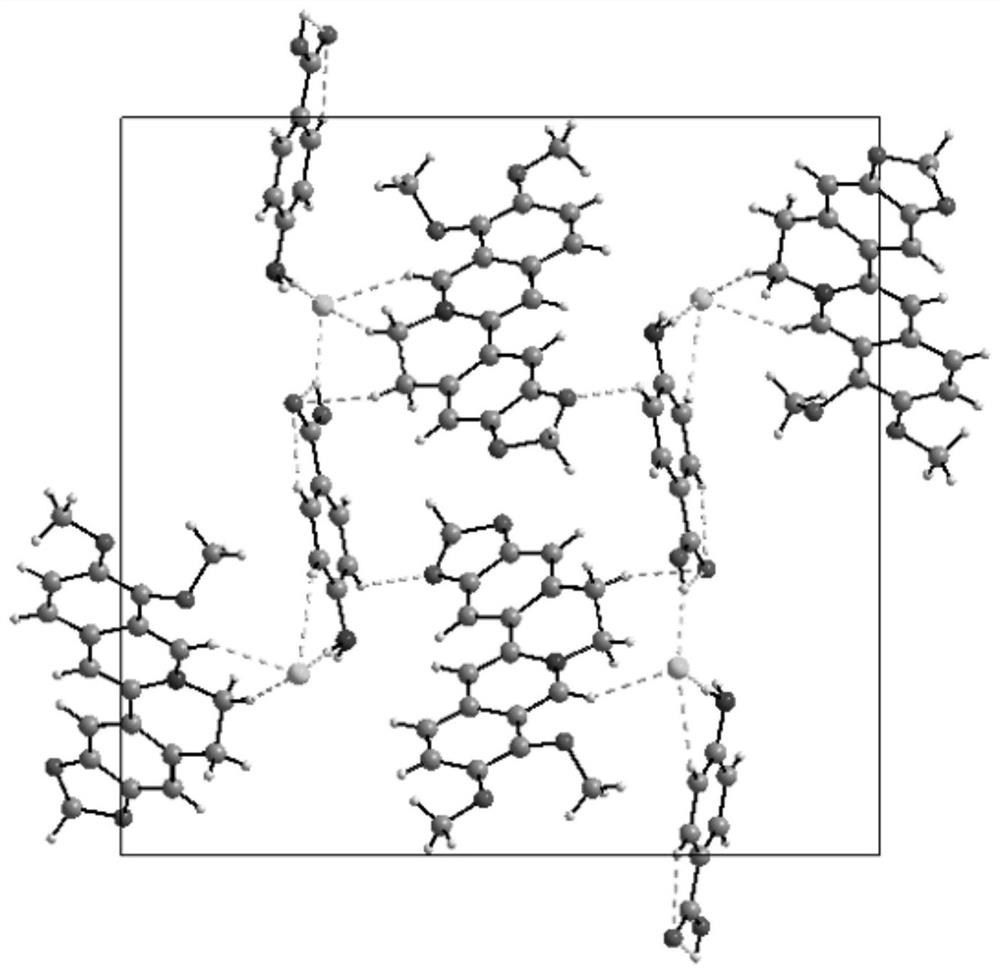
Berberine hydrochloride pharmaceutical co-crystal as well as preparation method and application thereof
A technology of berberine hydrochloride and medicine, which is applied in the field of berberine hydrochloride drug co-crystal and its preparation, can solve problems such as poor treatment effect, achieve large commercial application value, realize large-scale industrial production, and have low production cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Add berberine hydrochloride (100mg) and 4-aminobenzoic acid with a molar ratio of 1:1 into methanol, ultrasonicate, heat to 40°C, the solution is clear, continue ultrasonication for a period of time, take it out and let it stand at room temperature for 48 hours After the precipitation is complete, filter and dry to obtain light yellow solid powder, which is berberine hydrochloride-4-aminobenzoic acid eutectic; wherein, the total mass g of berberine hydrochloride and 4-aminobenzoic acid and the volume of methanol The ml ratio is 1:50–100.
Embodiment 2
[0059] Add berberine hydrochloride (100mg) and 4-aminobenzoic acid with a molar ratio of 1:1 into ethanol, ultrasonicate, heat to 50°C, the solution is clear, continue ultrasonication for 10min, take it out and let it stand at room temperature for 48h to precipitate After complete filtration and drying, a light yellow solid powder is obtained, which is the co-crystal of berberine hydrochloride-4-aminobenzidine; wherein, the total mass g of berberine hydrochloride and 4-aminobenzidine and the volume ml of ethanol The ratio is 1:100–150.
Embodiment 3
[0061] Add berberine hydrochloride (100mg) and 4-aminobenzoic acid with a molar ratio of 1:1 into isopropanol, ultrasonicate, heat to 60°C, the solution is clear, continue ultrasonication for 10min, take it out and let it stand at room temperature, After 72 hours of complete precipitation, filter and dry to obtain light yellow solid powder, which is berberine hydrochloride-4-aminobenzoic acid eutectic; wherein, the total mass g of berberine hydrochloride and 4-aminobenzoic acid and isopropyl The volume ml ratio of alcohol is 1:150–200.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com