Preparation method of visible-light response high-activity TiO2 composite photocatalyst
A catalyst and visible light technology, used in chemical instruments and methods, physical/chemical process catalysts, chemical/physical processes, etc., can solve problems such as low quantum efficiency and achieve the effect of performance enhancement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
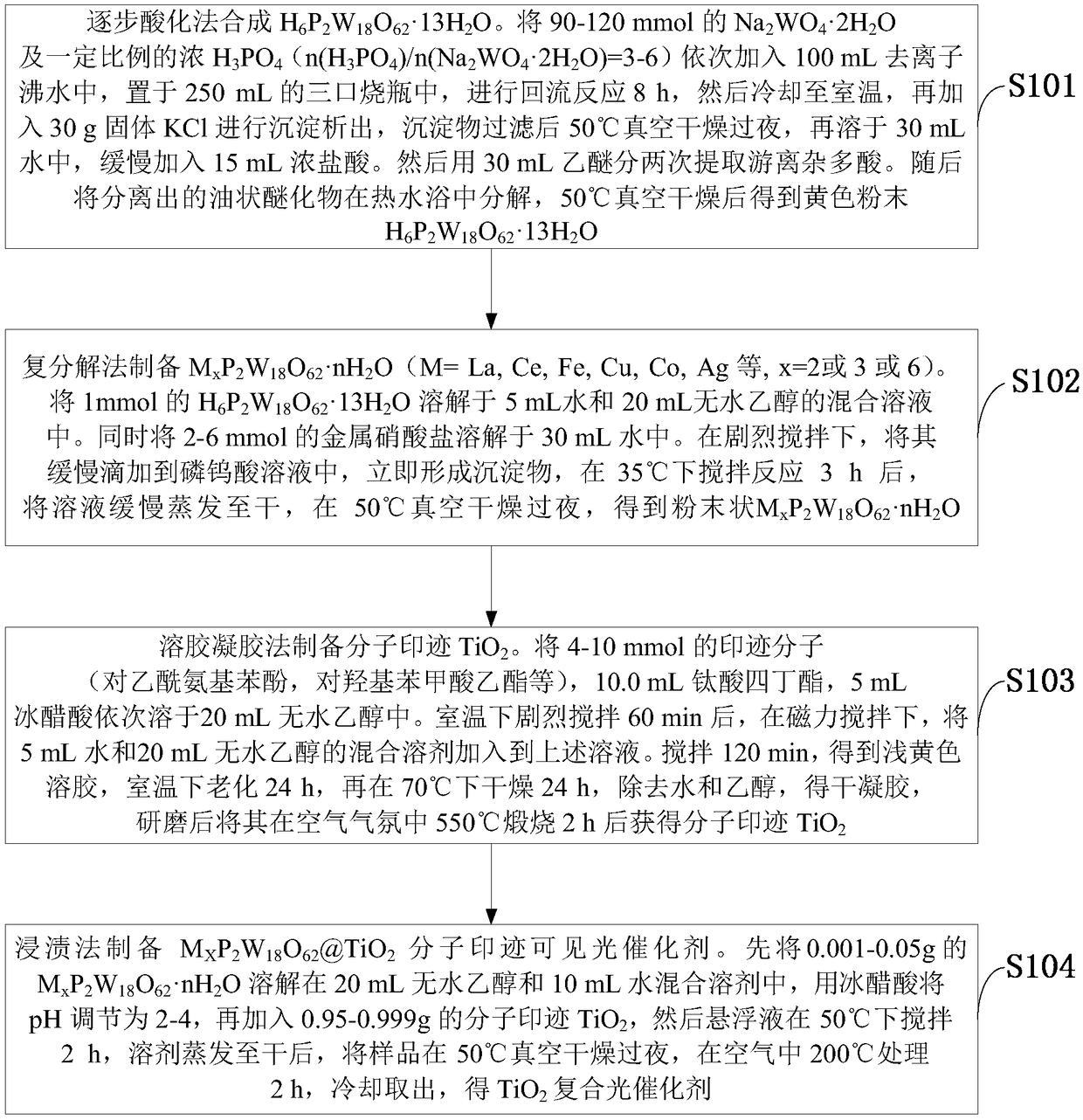
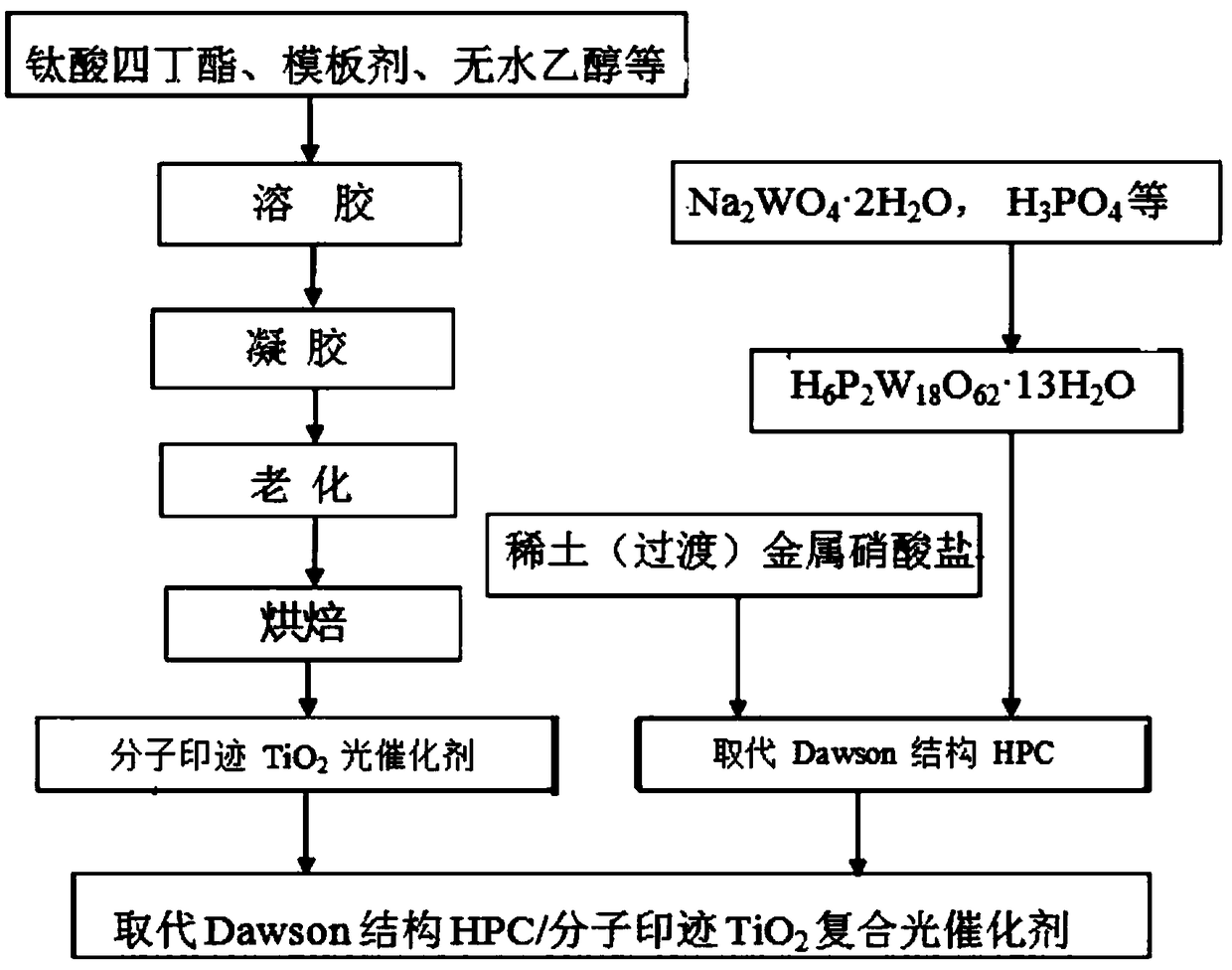
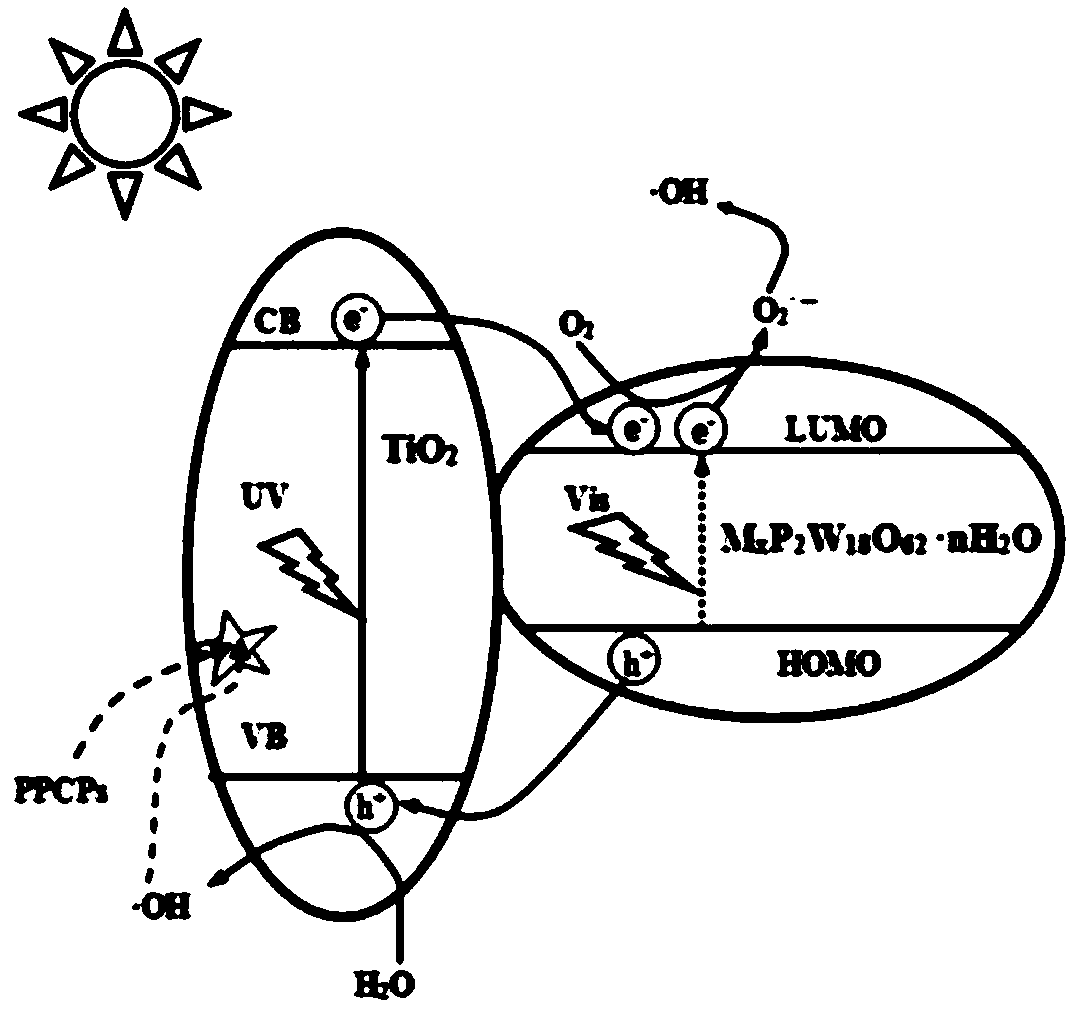
[0022] Such as figure 1 As shown, the visible light response high activity TiO provided by the embodiment of the present invention 2 The preparation method of composite photocatalyst comprises the following steps:
[0023] S101: Synthesis of H by stepwise acidification 6 P2 W 18 o 62 13H 2 O. 90-120mmol of Na 2 WO 4 2H 2 O and a certain proportion of concentrated H 3 PO 4 (n(H 3 PO 4 ) / n(Na 2 WO 4 2H 2 (0)=3-6) Add 100mL of deionized boiling water in turn, place in a 250mL three-neck flask, carry out reflux reaction for 8h, then cool to room temperature, then add 30g of solid KCl for precipitation, and vacuum dry the precipitate at 50°C after filtration Overnight, redissolve in 30mL of water, and slowly add 15mL of concentrated hydrochloric acid. Then use 30mL ether to extract the free heteropolyacid twice. Then decompose the separated oily ether compound in a hot water bath and dry it under vacuum at 50°C to obtain a yellow powder H 6 P 2 W 18 o 62 13H 2...
Embodiment 1
[0030] (1) 30g Na 2 WO 4 2H 2 O, 45 mL concentrated H 3 PO 4 Add 100mL of deionized boiling water in turn, place in a 250mL flask, carry out reflux reaction for 8h, then cool to room temperature, then add 30g of solid KCl for precipitation, the precipitate is filtered and vacuum-dried at 50°C overnight, then dissolved in 30mL of water, Slowly add 15mL of concentrated hydrochloric acid. Then use 30mL ether to extract the free heteropolyacid twice. Then decompose the separated oily ether compound in a hot water bath and dry it under vacuum at 50°C to obtain a yellow powder H 6 P 2 W 18 o 62 13H 2 O.
[0031] (2) Add 1mmol of H 6 P 2 W 18 o 62 13H 2 O was dissolved in a mixed solution of 5 mL of water and 20 mL of absolute ethanol. At the same time, 3 mmol of metal copper nitrate was dissolved in 30 mL of water. Under vigorous stirring, it was slowly added dropwise to the phosphotungstic acid solution, and a precipitate was formed immediately. After stirring and ...
Embodiment 2
[0035] (1)H 6 P 2 W 18 o 62 13H 2 The preparation of O is the same as in Example 1.
[0036] (2) Add 1mmol of H 6 P 2 W 18 o 62 13H 2 O was dissolved in a mixed solution of 5 mL of water and 20 mL of absolute ethanol. At the same time, 2 mmol of lanthanum nitrate was dissolved in 30 mL of water. Under vigorous stirring, it was slowly added dropwise to the phosphotungstic acid solution, and a precipitate was formed immediately. After stirring and reacting at 35°C for 3h, the solution was slowly evaporated to dryness, and dried in vacuum at 50°C overnight to obtain powdered La 2 P 2 W 18 o 62 ·nH 2 O.
[0037] (3) Dissolve 5mmol of ethyl p-hydroxybenzoate, 10.0mL of tetrabutyl titanate, and 5mL of glacial acetic acid in 20mL of absolute ethanol in sequence, and after stirring vigorously at room temperature for 60 minutes, under magnetic stirring, dissolve 5mL of water and 20mL of A mixed solvent of water and ethanol was added to the above solution. Stir for 120m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com