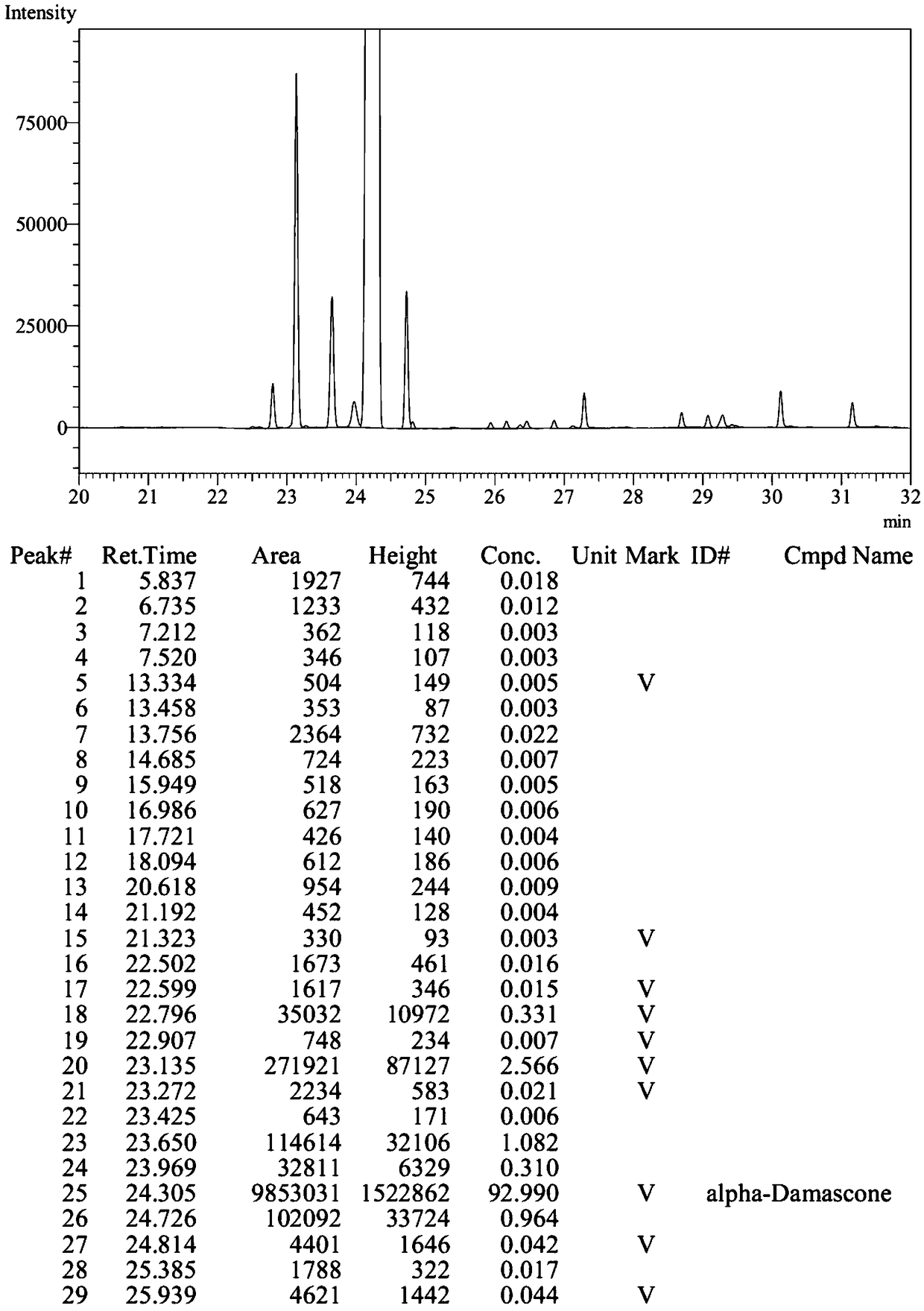
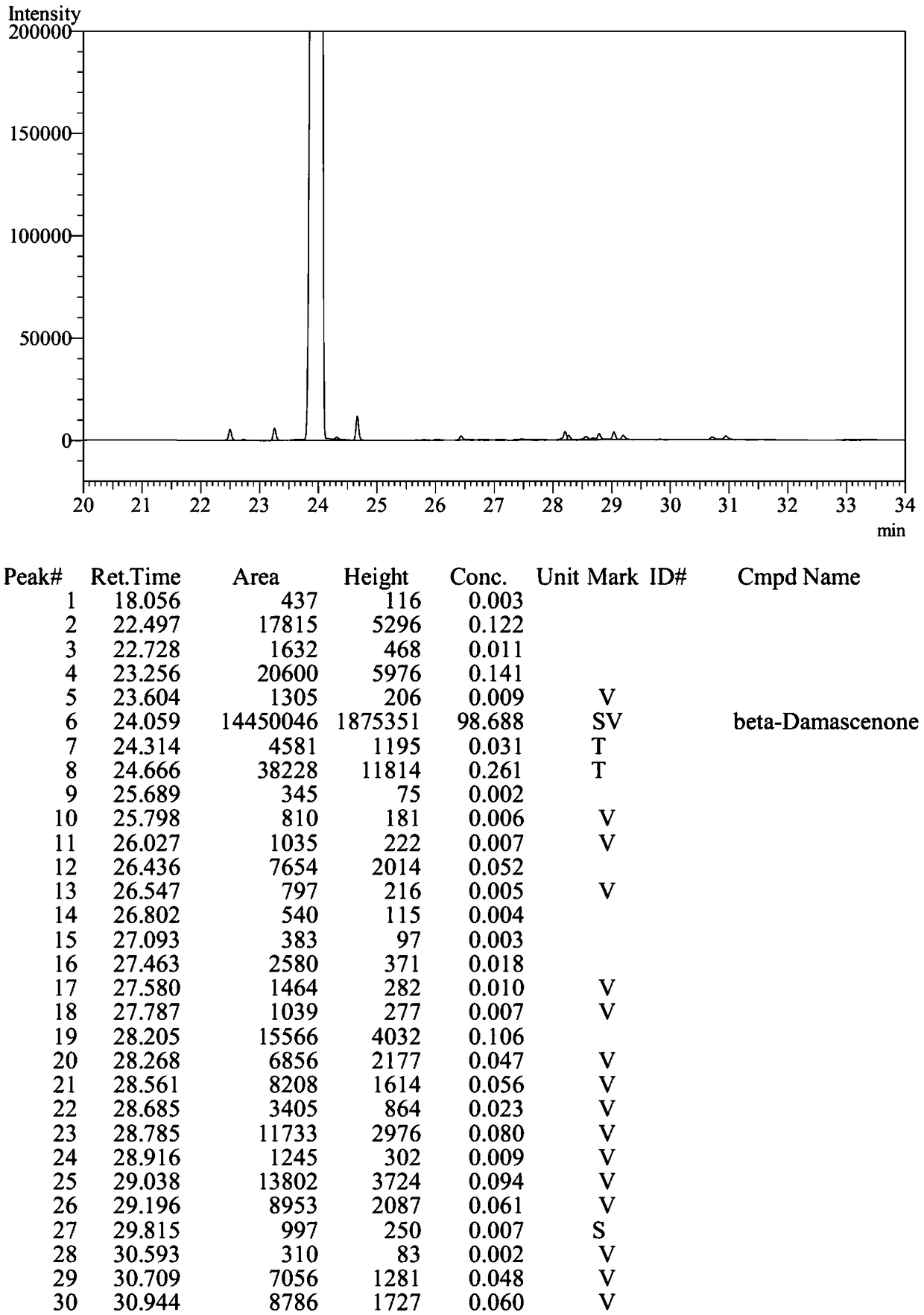
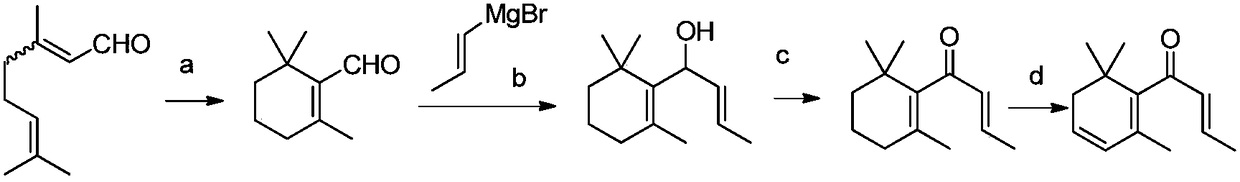
Method for synthesizing beta-damascenone
A technology of tukelenone and tukelenone, which is applied in the field of synthesizing β-tekelenone, can solve problems such as high safety risks, and achieve the effects of simple process operation, low cost and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0047] Below by embodiment the content of the present invention is specified:
[0048] 1) Synthesis of α-ringgeranic acid:
[0049] Step 1: Using citral as a raw material, an intermediate of geranic acid is obtained through an improved Pinnick oxidation reaction. specifically:
[0050] Add citral (100g), dipentene (446g), and tert-butanol (486g) to a 5L three-necked bottle, add 902g of 16.8% sodium dihydrogen phosphate aqueous solution, and slowly add 20% of it dropwise at 15°C. Sodium chlorite solution 379.6g, the temperature is not higher than 35°C; after the dropwise addition, stir and react at room temperature for 16h; when the raw materials are completely reacted, separate the liquid, separate the upper organic phase, and extract the lower aqueous phase with toluene (2x100mL). The organic phases were combined, and tert-butanol and toluene were removed by rotary evaporation; then 400 g of 10% sodium hydroxide solution was stirred for 0.5 h, separated, the aqueous phase w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com