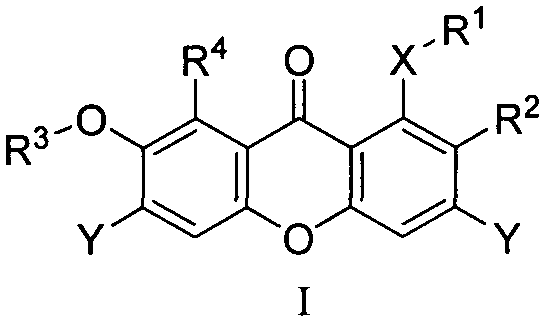
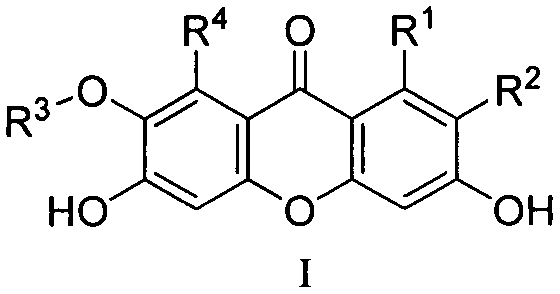
Phosphoglyceromutase 1 inhibitor and application in related diseases thereof
A technology of phosphoglycerate and mutase, applied in organic chemistry, drug combination, antineoplastic drugs, etc., can solve the problems of poor cell activity, unclear structure-activity relationship, toxic and side effects of anthraquinone skeleton structure, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
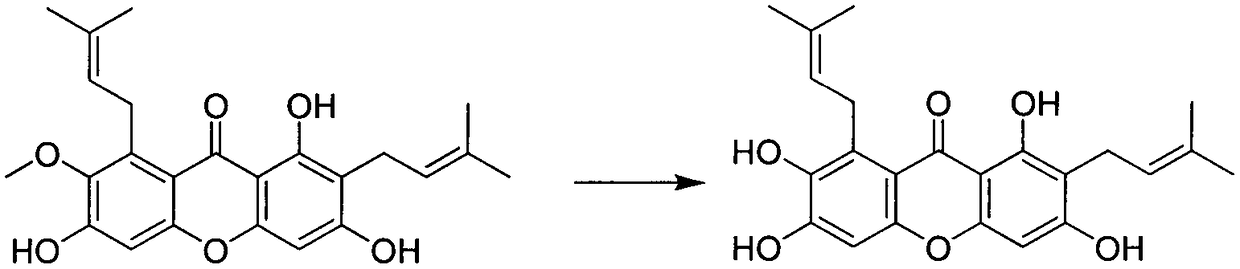
[0026] Embodiment 1 Synthesis of some compounds of the present invention
[0027] Preparation of 1,3,6,7-tetrahydroxy-2,8-di(3-methylbut-2-en-1-yl)-xanthone (Ib)
[0028]
[0029] Dissolve 1,3,6-trihydroxy-7-methoxy-2,8-bis(3-methyl-2-butenyl)-xanthone (Ia) (410 mg, 1.0 mmol) in 10 mL of DMF , added NaH (480mg, 10.0mmol), stirred at room temperature under nitrogen protection for 20min, added ethanethiol (1.4mL, 20.0mmol), heated to reflux for 5h, TLC detection, the raw materials were completely reacted, cooled to room temperature, added 20mL of water and 60mL of acetic acid Ethyl ester, extraction, the organic phase was washed three times with saturated ammonium chloride, and the organic phase was dried over anhydrous sodium sulfate. Purified by column chromatography (dichloromethane:methanol=30:1→15:1) to obtain 214 mg of light yellow solid, yield: 54%. 1 H-NMR (300MHz, CDCl 3 ): δ13.90(s, 1H), 11.16(s, 1H), 10.72(s, 1H), 8.63(s, 1H), 6.76(s, 1H), 6.33(s, 1H), 5.20-5.18...
Embodiment 2
[0059] Example 2 Compounds Ia-Ii inhibit PGAM1 activity experiment.
[0060] The invention measures the inhibitory activity of the small molecule inhibitor on PGAM1 based on an enzyme-linked method. Using 3PG as a substrate, it is finally converted into lactic acid through the joint action of four enzymes, PGAM1, enolase, pyruvate kinase (PK) and lactate dehydrogenase (LDH). In the last step of the reaction, a molecule of NADH is oxidized to generate NAD+, and the inhibitory activity of the small molecule on PGAM1 is determined by detecting the signal change of NADH (λ=340nm). The enzyme-linked reaction involves 4 enzymes. In order to verify the specificity and specificity of small molecule compounds, 2PG is used as a substrate, and enolase, PK and LDH are used as catalyzed enzymes to determine the reaction rate as a negative control. Possibility of false-positive small molecule compounds that inhibit the latter three target enzymes.
[0061] experimental method
[0062] Ad...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com