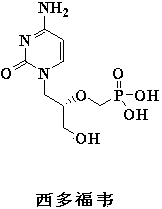
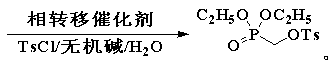Synthesis method of diethyl (tosyloxy)methylphosphonate
A technology of diethyl toluenesulfonyloxymethylphosphonate and p-toluenesulfonyl chloride, which is applied in the field of medicine and chemical industry, can solve the problems of cumbersome operation, many impurities, and high cost, and achieve the reduction of side reactions, high product yield, and improved Yield and Quality Effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1 Synthesis of diethyl p-toluenesulfonyloxymethylphosphonate
[0035]34.5 g (1.15 mol) of paraformaldehyde, 84.8 g (0.80 mol) of sodium carbonate and 0.72 g (2.6 mmol) of tetrabutylammonium chloride were dissolved in 900 mL of water, and 138.0 g (1.0 mol) of phosphorous acid was added dropwise under good stirring For diethyl ester, the dropwise addition process was maintained at 5-10°C, the drop was completed within 4 hours, the temperature was raised to 80°C within 1.5 hours, and the temperature was kept at 80-85°C for 10 hours. After the condensation is completed, cool to below 3°C, add 217.2 g (1.14 mol) p-toluenesulfonyl chloride in small batches, keep at 3-8°C during the addition process, finish adding in 4 hours, raise the temperature to 30°C within 1.5 hours, ℃ insulation 9h. After the esterification, it was cooled to below 16°C, allowed to stand and separated, and 307.2 g of diethyl p-toluenesulfonyloxymethylphosphonate was isolated, with a yield of 95....
Embodiment 2
[0036] Example 2 Synthesis of diethyl p-toluenesulfonyloxymethylphosphonate
[0037] 39.0 g (1.30 mol) of paraformaldehyde, 68.9 g (0.65 mol) of sodium carbonate and 2.86 g (10.5 mmol) of benzyltriethylammonium bromide were dissolved in 1800 mL of water, and 138.0 g (1.0 mol) was added dropwise under good stirring For diethyl phosphite, the dropping process was maintained at 3-8°C, the drop was completed within 5 hours, the temperature was raised to 78°C within 2.5 hours, and the temperature was kept at 78-83°C for 14 hours. After the condensation is completed, cool to below 5°C, add 247.6 g (1.30 mol) p-toluenesulfonyl chloride in small batches, keep at 5-10°C during the feeding process, finish adding in 3 hours, heat up to 48°C within 4.5 hours, and keep at 48-53 ℃ insulation 3.5h. After the esterification, it was cooled to below 18°C, allowed to stand and separated, and 302.4 g of diethyl p-toluenesulfonyloxymethylphosphonate was isolated, with a yield of 93.9% and a conte...
Embodiment 3
[0038] Example 3 Synthesis of diethyl p-toluenesulfonyloxymethylphosphonate
[0039] 36.6 g (1.22 mol) of paraformaldehyde, 154.6 g (1.12 mol) of potassium carbonate and 3.14 g (7.0 mmol) of methyl trioctyl ammonium bromide were dissolved in 2500 mL of water, and 138.0 g (1.0 mol) was added dropwise under good stirring For diethyl phosphite, the dropping process was maintained at 0-5°C, and the drop was completed within 10 hours. The temperature was raised to 90°C within 4 hours, and kept at 90-95°C for 4 hours. After the condensation is completed, cool to below 5°C, add 257.2 g (1.35 mol) p-toluenesulfonyl chloride in small batches, keep at 5-10°C during the addition process, finish adding in 6 hours, raise the temperature to 33°C within 2.5 hours, at 33-38 ℃ insulation 4h. After the esterification, it was cooled to below 20°C, allowed to stand and separated, and 300.8 g of diethyl p-toluenesulfonyloxymethylphosphonate was isolated, with a yield of 93.4% and a content of 98....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com