Mn doped Ni3S2 nanoarray hydrogen evolution catalyst and preparation method and application thereof
A nano-array and nano-sheet array technology is applied in the field of Mn-doped Ni3S2 nano-array hydrogen evolution catalyst and its preparation, which can solve the problems of small reserves, high cost and the like, and achieve high electrochemical conductivity, good durability, and high surface area. The effect of roughness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
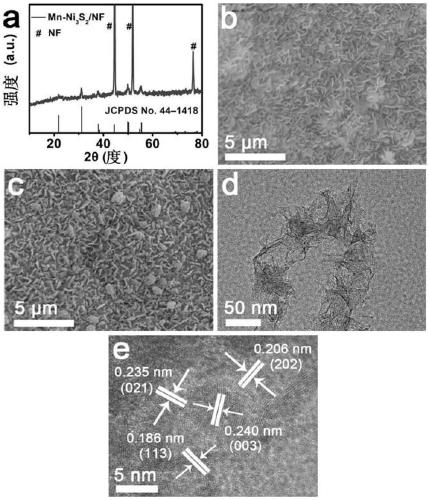
[0027] (1) Preparation of precursor by hydrothermal method: ① 1mmol Ni(NO 3 ) 2 ·6H 2 O, 0.2mmol Mn(NO 3 ) 2 , 10mmol urea was put into 40mL deionized water and stirred until the solution was clear. ② Transfer the pretreated nickel foam and the clear solution prepared in ① to a polytetrafluoroethylene autoclave, keep it warm at 120°C for 6.5h, and cool to room temperature Take out, rinse with water to obtain the precursor;
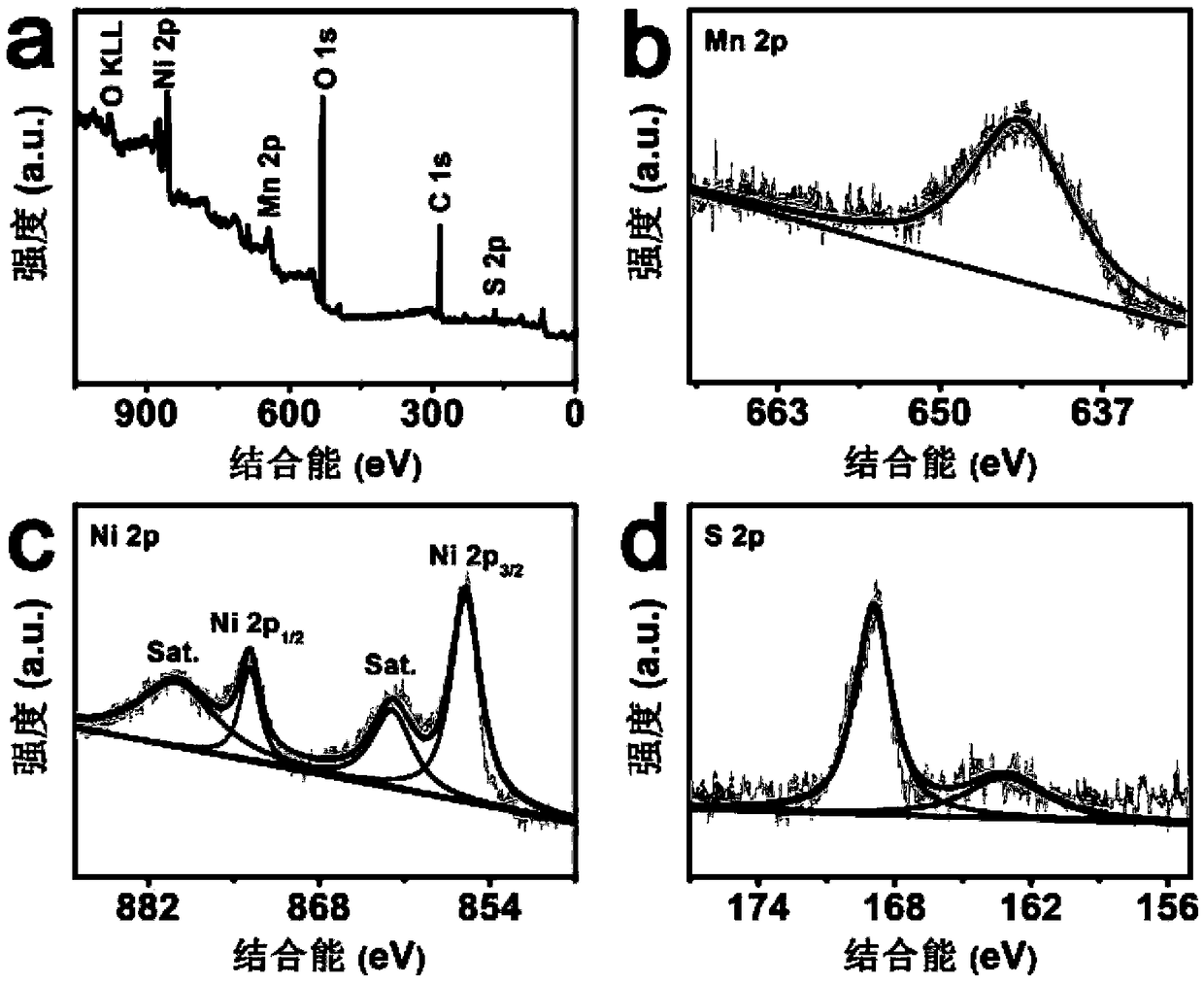
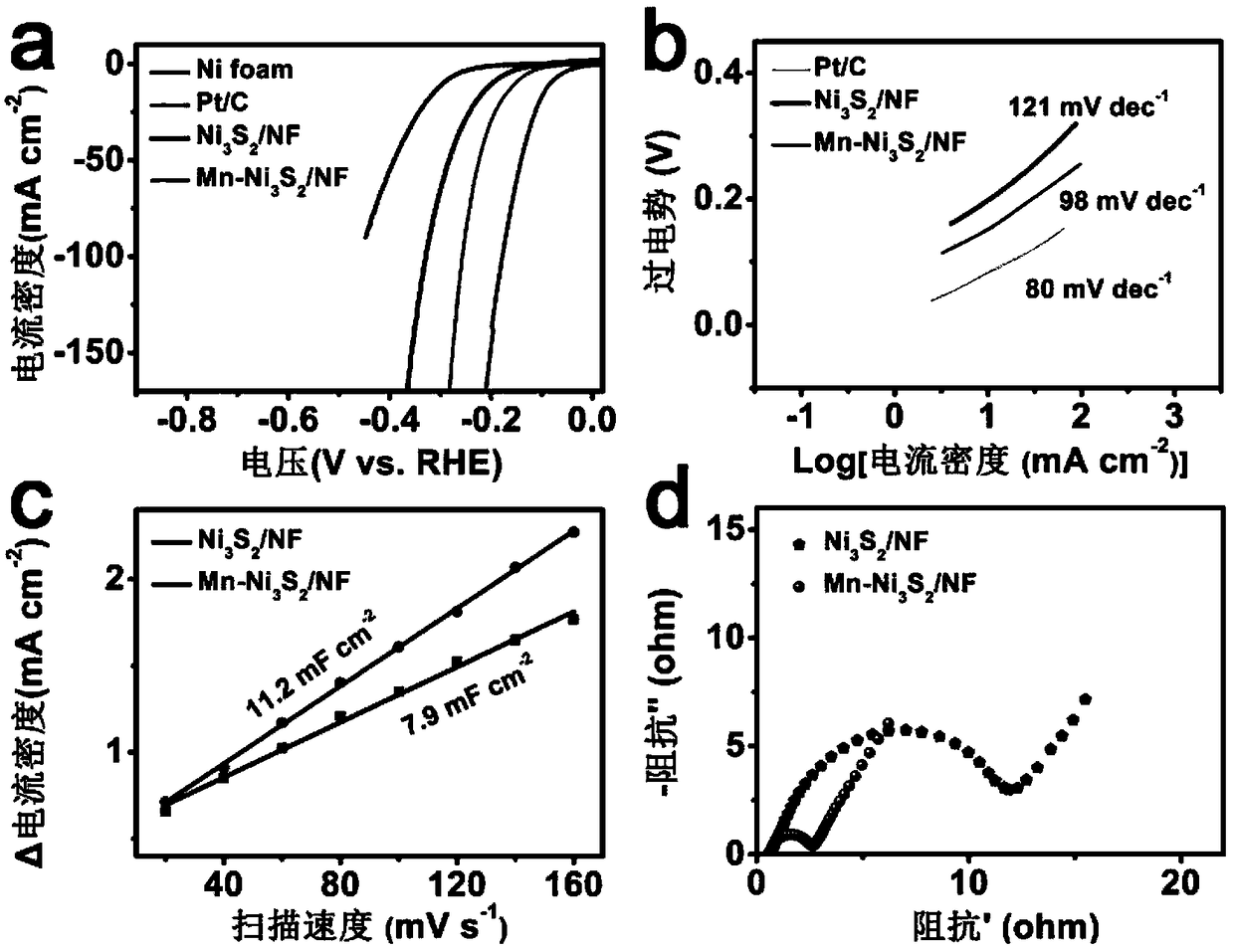
[0028] (2) Preparation of Mn-Ni 3 S 2 / NF: Add 8mmol of Na 2 S was dissolved in 40mL deionized water, and the precursor prepared in step (1) and Na 2 The S solution was transferred into a polytetrafluoroethylene autoclave, kept at 120°C for 3.5h, cooled to room temperature, taken out, rinsed with water, and prepared Mn-Ni 3 S 2 / NF, nanosheet array (Mn-Ni 3 S 2 ) on nickel foam (NF) with a loading of 1.1mg cm -2 .
Embodiment 2
[0030] (1) Preparation of precursor by hydrothermal method: ① 1mmol Ni(NO 3 ) 2 ·6H 2 O, 0.1mmol Mn(NO 3 ) 2 , 10mmol urea was put into 40mL deionized water and stirred until the solution was clear. ② Transfer the pretreated nickel foam and the clear solution prepared in ① to a polytetrafluoroethylene autoclave, keep it warm at 125°C for 6h, and take it out after cooling to room temperature. , washing with water to prepare the precursor;
[0031] (2) Preparation of Mn-Ni 3 S 2 / NF: Add 8mmol of Na 2 S was dissolved in 55mL deionized water, and the precursor prepared in step (1) and Na 2 The S solution was transferred into a polytetrafluoroethylene autoclave, kept at 110°C for 4 hours, cooled to room temperature, taken out, and washed with water to obtain Mn-Ni 3 S 2 / NF, nanosheet array (Mn-Ni 3 S 2 ) on nickel foam (NF) is 1.2mg·cm -2 .
Embodiment 3
[0033](1) Preparation of precursor by hydrothermal method: ① 1mmol Ni(NO 3 ) 2 ·6H 2 O, 0.3mmol Mn(NO 3 ) 2 , 10mmol urea was put into 40mL deionized water and stirred until the solution was clear. ② Transfer the pretreated nickel foam and the clear solution prepared in ① to a polytetrafluoroethylene autoclave, keep it warm at 115°C for 7h, and take it out after cooling to room temperature. , washing with water to prepare the precursor;
[0034] (2) Preparation of Mn-Ni 3 S 2 / NF: Add 8mmol of Na 2 S was dissolved in 40mL deionized water, and the precursor prepared in step (1) and Na 2 The S solution was transferred into a polytetrafluoroethylene autoclave, kept at 125°C for 3 hours, cooled to room temperature, taken out, and rinsed with water to obtain Mn-Ni 3 S 2 / NF, nanosheet array (Mn-Ni 3 S 2 ) on nickel foam (NF) is 1.2mg·cm -2 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com