Method for treating brain atrophy
A brain atrophy and uridine technology, applied in the field of medical nutrition, can solve the problems that the nutritional intake of subjects at risk of neurodegenerative diseases has not been fully studied, and the increase of subjects with brain atrophy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
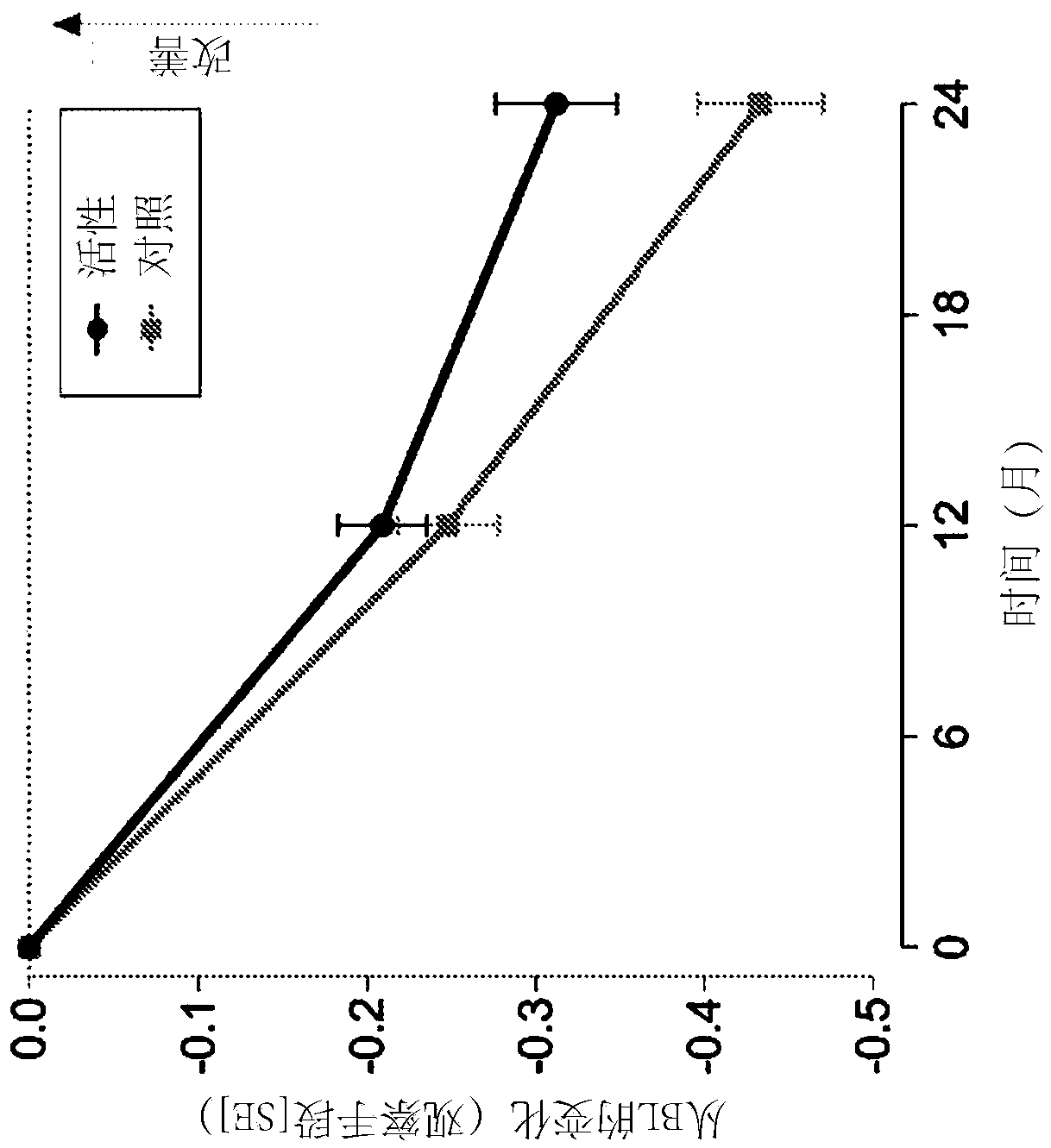
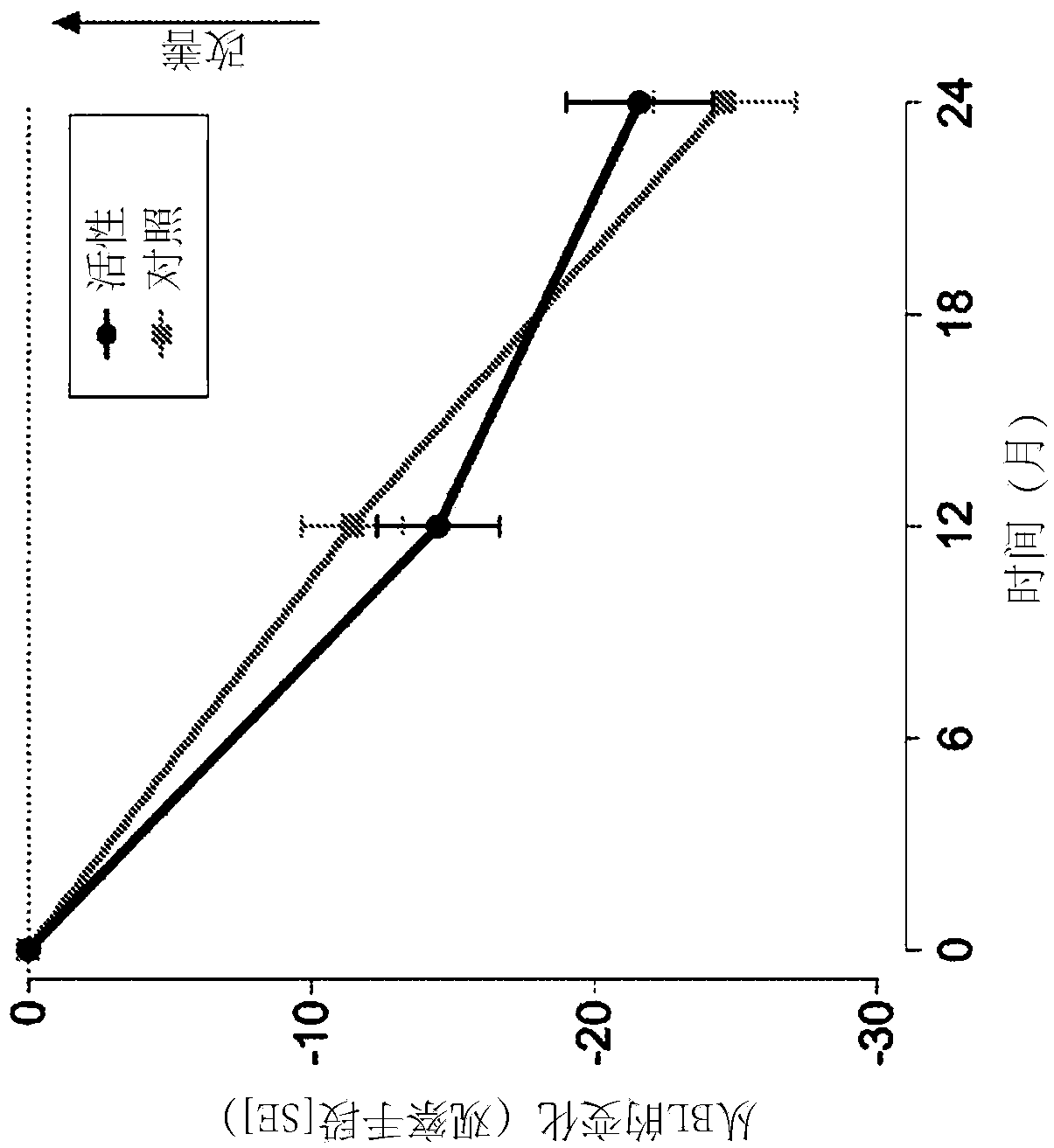
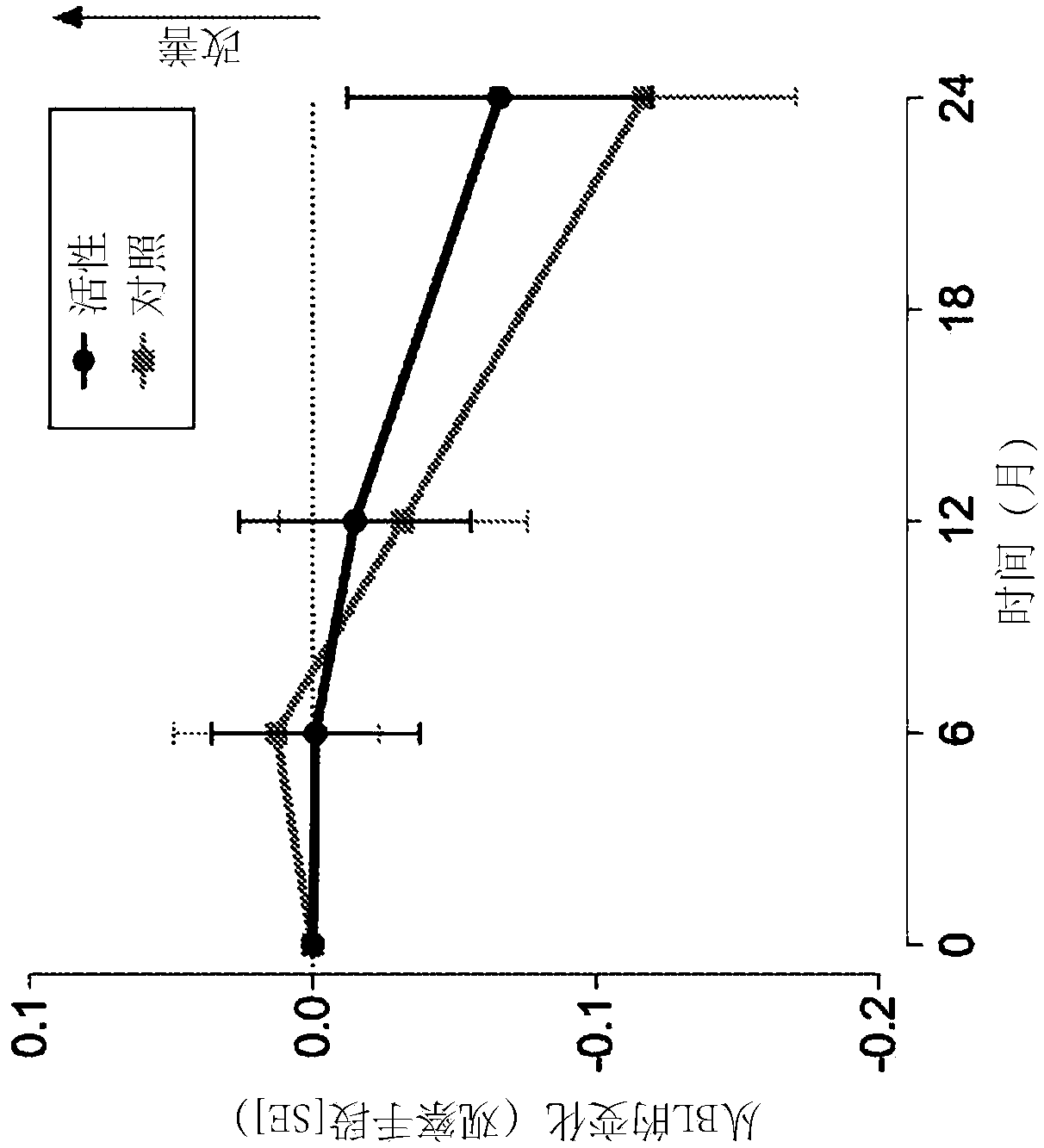
[0072] Example 1. Clinical Trials
[0073] Carry out randomized, controlled, double-blind, parallel group, multi-national clinical research, wherein 311 prodromal period subjects use composition of the present invention (active composition, every 125ml: 300mg EPA, 1200mg DHA, 106mg phospholipid, 400mg choline, 625mg UMP, 40mg vitamin E, 80mg vitamin C, 60mcg selenium, 3mcg vitamin B12, 1mg vitamin B6, 400mcg folic acid) for 2 years or receive isocaloric control.
[0074] Inclusion criteria for prodromal patients were defined as follows:
[0075] 1) Suffering from episodic memory impairment defined as -1 SD on 2 out of 8 tests (explained further below) with at least a memory test score of -1 SD.
[0076] 2) Evidence of underlying Alzheimer's disease pathology
[0077] - Medial temporal lobe atrophy ≥1 as determined by MRI images
[0078] - Cerebrospinal fluid measurement: beta-amyloid ratio 60 or t-tau >350, or,
[0079] - Abnormal FDG-PET matching changes in Alzheimer's ...
Embodiment 2
[0102] Example 2. Effects of Healthy Rats Exposure to Compositions of the Invention on Synapses
[0103] The added value of vitamin C, vitamin E and selenium according to the invention was evaluated in healthy rats supplemented with uridine (as uridine-5'-monophosphate) and fish oil (FO) containing DHA and EPA.
[0104] Rats were randomized into 4 treatment groups and fed with 1 of 4 intervention diets for 6 weeks (see Table 1). After completing the diet treatment, the rats were sacrificed, and the brain samples were analyzed for phospholipids, synaptic proteins, and 2 enzymes involved in phospholipid synthesis (i.e., choline phosphoryl cytidine transferase (PCYT1A) and choline / ethanolamine phosphotransferase (CEPT1A). ))s level.
[0105] Table 1: Diets containing different amounts of antioxidants, fish oil and UMP.
[0106]
[0107]
[0108] The levels of total and individual phospholipids (except phosphatidylinositol), the levels of the presynaptic protein Synapsin-1...
Embodiment 3
[0113] Example 3: In Vitro Study of the Effects of the Compositions of the Invention on Neuronal Growth
[0114] PC12 pheochromocytoma cells were grown at 37°C in a humidified atmosphere with 95% air and 5% carbon dioxide in DMEM (Gibco) supplemented with 10% fetal bovine serum (FBS), penicillin (100 units / ml ) and streptomycin (100 μg / ml) (Gibco). The cells were seeded in a 96-well plate at a density of 2000 cells / well, and the culture medium containing 20 ng / ml nerve growth factor (NGF) was supplemented 24 hours later. Cells were exposed to the composition of the invention or left without supplementation (control). The composition of the cell supplementation is shown in Table 3. Cell replenishment was performed three times. These conditions were compared to unsupplemented cells. After supplementing with these conjugates for 2 days, cells were stained with Calcein-AM stain (2 ng / µl) in 100 µl of medium per well and nuclei were counterstained with Hoechst stain (0.6 µg / m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com