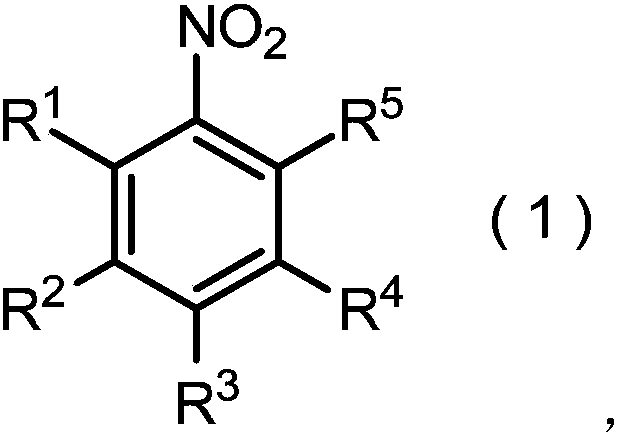
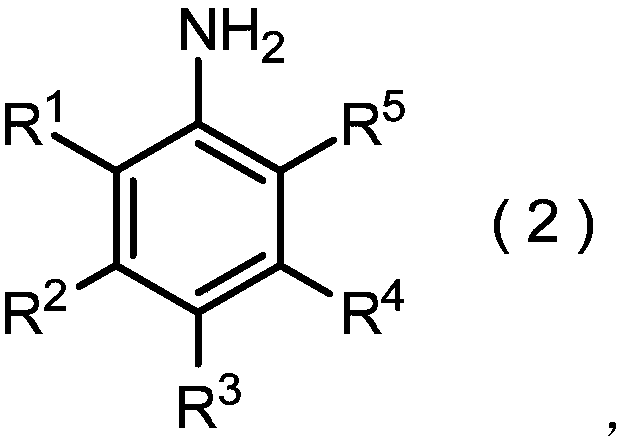
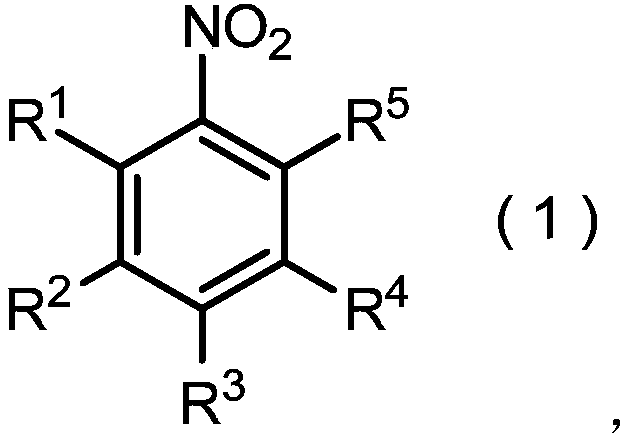
Method for producing nitrobenzene compound
The technology of a compound, nitrobenzene, is applied in the field of manufacture of nitrobenzene compounds to achieve the effect of reducing the amount of waste, excellent yield, and improving safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0251] Manufacture of 2,6-Dichloronitrobenzene Using Nitric Acid
[0252] Add 2,6-dichloroaniline 16.2g (0.10mol), water 81.7mL and 69% nitric acid 21g (0.23mol) in the 200mL four-neck flask equipped with stirrer, reflux condenser, thermometer and dropping funnel, the mixture Heat at 60°C to dissolve. The mixture was cooled to 37°C while stirring, and after stirring at this temperature for 30 minutes, the mixture was further cooled to 0°C. After 19.0 g (0.11 mol) of a 40% sodium nitrite aqueous solution was added dropwise thereto at 0-5°C over 25 minutes, the reaction mixture was stirred at 0-5°C for 30 minutes. Thereafter, 36 mL of a 5% aqueous sodium bicarbonate solution was added dropwise to adjust the pH of the reaction mixture to 0.6-1.3, and then 17.5 mL of toluene and 30 g of water were added. After the mixture obtained above was partitioned into a toluene layer and an aqueous layer, the aqueous layer was separated.
Embodiment 2
[0255] Manufacture of 2,6-Dichloronitrobenzene Using Nitric Acid
[0256] Add 32.4g (0.20mol) of 2,6-dichloroaniline, 190mL of water and 42.0g (0.46mol) of 69% nitric acid in a 500mL four-necked separable flask equipped with a stirrer, reflux condenser, thermometer and dropping funnel , and the mixture was dissolved by heating at 60°C. The mixture was cooled to 37°C while stirring, and after stirring at this temperature for 30 minutes, the mixture was further cooled to 0°C. After 38.0 g (0.22 mol) of 40% sodium nitrite aqueous solution was added dropwise thereto at -3°C (minus 3°C) to 5°C over 35 minutes, the reaction mixture was stirred at -3°C to 5°C for 30 minutes. Thereafter, 72.2 mL of a 5% aqueous sodium bicarbonate solution was added dropwise to adjust the pH of the reaction mixture to 0.8-2.0, and then 30.4 mL of toluene was added. After the mixture obtained above was partitioned into a toluene layer and an aqueous layer, the aqueous layer was separated. The resulti...
Embodiment 3
[0294] Manufacture of methyl 3-chloro-2-nitrobenzoate using nitric acid
[0295] Add 35% isobutyl methyl ketone (MIBK) solution 106.1g (0.20mol) of 2-amino-3-chlorobenzoic acid methyl ester in the 300mL four-neck flask equipped with stirrer, reflux condenser, thermometer and dropping funnel and Water 96.8mL. While stirring the mixture, 42 g (0.46 mol) of 69% nitric acid was added dropwise over 30 minutes, and then cooled to -5°C to 0°C while stirring. After 39.9 g (0.22 mol) of a 38% sodium nitrite aqueous solution was added dropwise thereto at -5°C to 0°C over 1 hour, the reaction mixture was stirred at -5°C to 0°C for 1 hour. After the mixture obtained as described above was partitioned into MIBK layer and aqueous layer, the aqueous layer was separated. The resulting aqueous layer was submitted to differential scanning calorimetry and accelerated calorimetry.
[0296] Add 9.7g (0.068mol) of copper (I) oxide, 22.5mL of water, 168mL of 10% aqueous sodium bicarbonate solutio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com