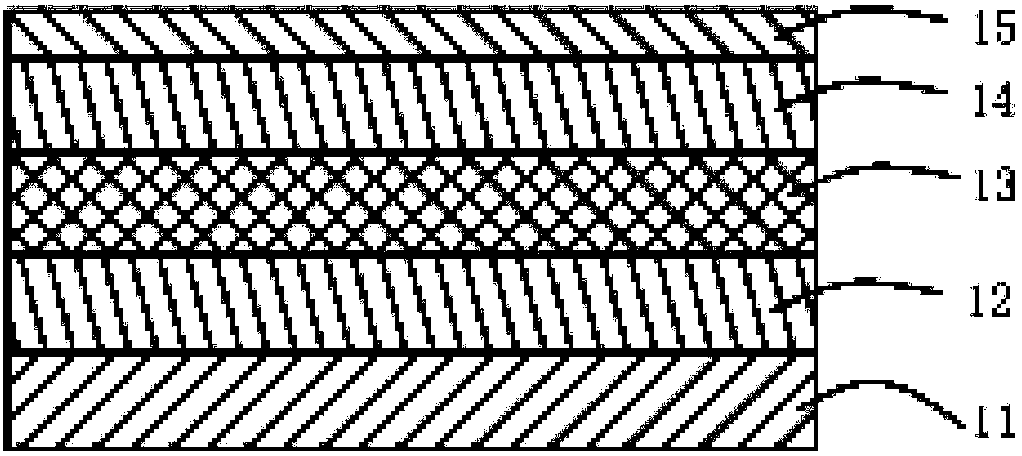
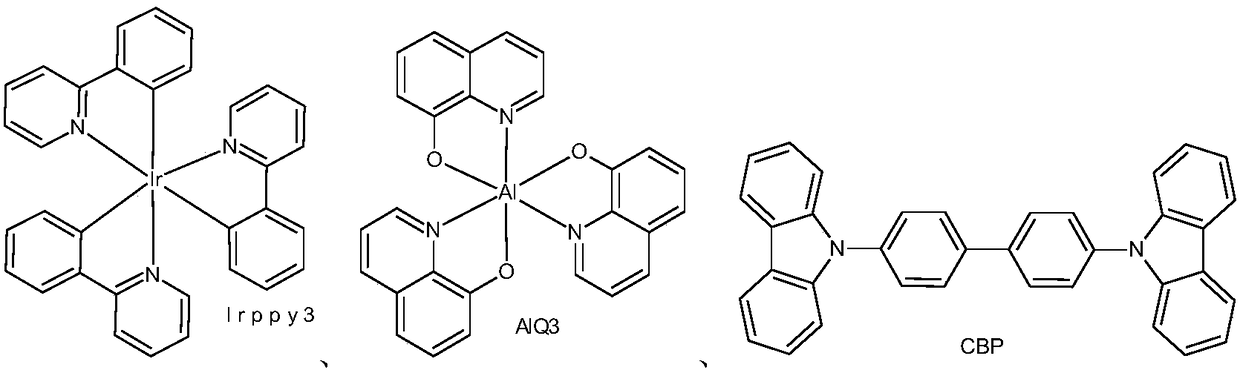
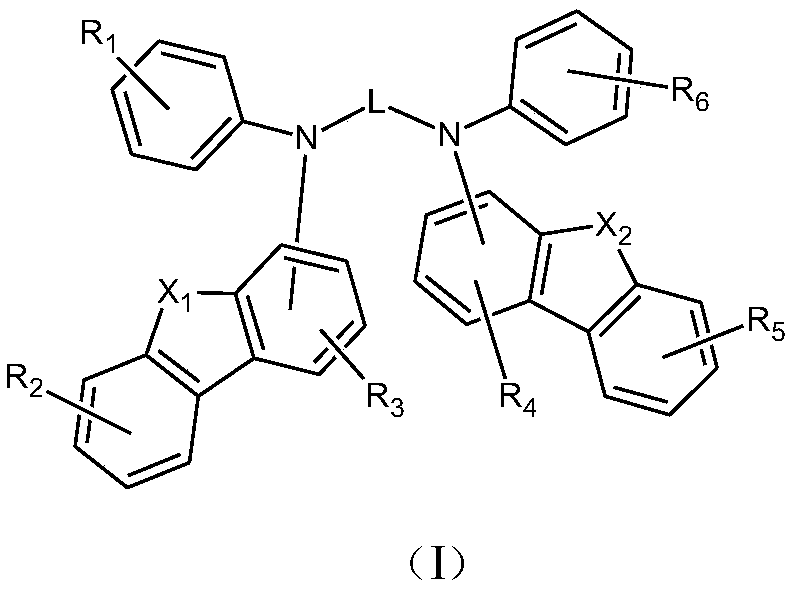
Organic semiconductor material and luminescent device
A technology of organic semiconductors and matrix materials, applied in semiconductor devices, semiconductor/solid-state device manufacturing, electric solid-state devices, etc., can solve problems such as the lack of auxiliary semiconductor materials, and achieve the effect of reducing turn-on voltage and improving efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0090] Preparation of organic semiconductor materials:
[0091] Method A: general synthetic route of general formula (I)
[0092] The method for preparing the compound of dibenzofuran structure, mixing dibenzofuran boronic acid and brominated aromatic compound in tetrahydrofuran, adding sodium carbonate and catalyst palladium acetate, heating and reacting for 24 hours, cooling, and extracting with dichloromethane, the column layer analysis and purification to obtain aryl-substituted dibenzofuran, and then react the obtained aryl-substituted dibenzofuran with liquid bromine in the presence of butyllithium in low-temperature tetrahydrofuran for 12 hours, and post-treatment to obtain bromine-substituted dibenzofuran. Benzofuran compound, finally react bromine-substituted dibenzofuran compound with corresponding amine in palladium acetate, ligand tri-tert-butylphosphine tBu3P, base tBuONa for 24h in dry toluene, extract with dichloromethane after cooling , column chromatography p...
Embodiment 1
[0100] The synthesis of embodiment 1:S001
[0101]
[0102] Bromobenzene (10.0mmol), dibenzofuran-4-boronic acid (12.0mmol), palladium acetate (0.3mmol) and sodium carbonate (20.00mmol) were dissolved in 50mL of tetrahydrofuran, reacted at 65°C for 24 hours, cooled, added water and Dichloromethane, the organic layer was concentrated and column chromatographed to obtain an intermediate product (S001-3). S001-3 (5mmol), liquid bromine (6mmol), and butyllithium (5mmol) were dissolved in 30mL of tetrahydrofuran, reacted at -78°C for 12h, and worked up to obtain the intermediate product S001-4. Dissolve 2,6-dibromospirobifluorene (10mmol) and aniline (20mmol) in 100mL of dry toluene, add catalyst palladium acetate, ligand tBu3P, base tBuONa, react under nitrogen for 12h, cool with dichloro Methane extraction. Then column chromatography gave the intermediate product S001-7. Dissolve S001-4 (5mmol) and S001-7 (5mmol) in 50mL dry toluene, add palladium acetate, ligand tBu3P, base ...
Embodiment 2
[0103] Embodiment 2: the synthesis of S002
[0104]
[0105] Dissolve 2,6-dibromospirobifluorene (10mmol) and 4-aminobiphenyl (20mmol) in 100mL dry toluene, add catalyst palladium acetate, ligand tBu3P, base tBuONa, react under nitrogen for 12h, cool Then extract with dichloromethane. Then column chromatography gave the intermediate product S002-7. Dissolve S001-4 (5mmol) and S002-7 (5mmol) in 50mL dry toluene, add palladium acetate, ligand tBu3P, base tBuONa, react for 12h under nitrogen, extract with dichloromethane after cooling, and obtain after column chromatography Final product S002 (2.3 mmol), yield 46.0%. MS (ESI-TOF): The measured mass-to-nucleus ratio m / z is 1135.35 [M+].
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com