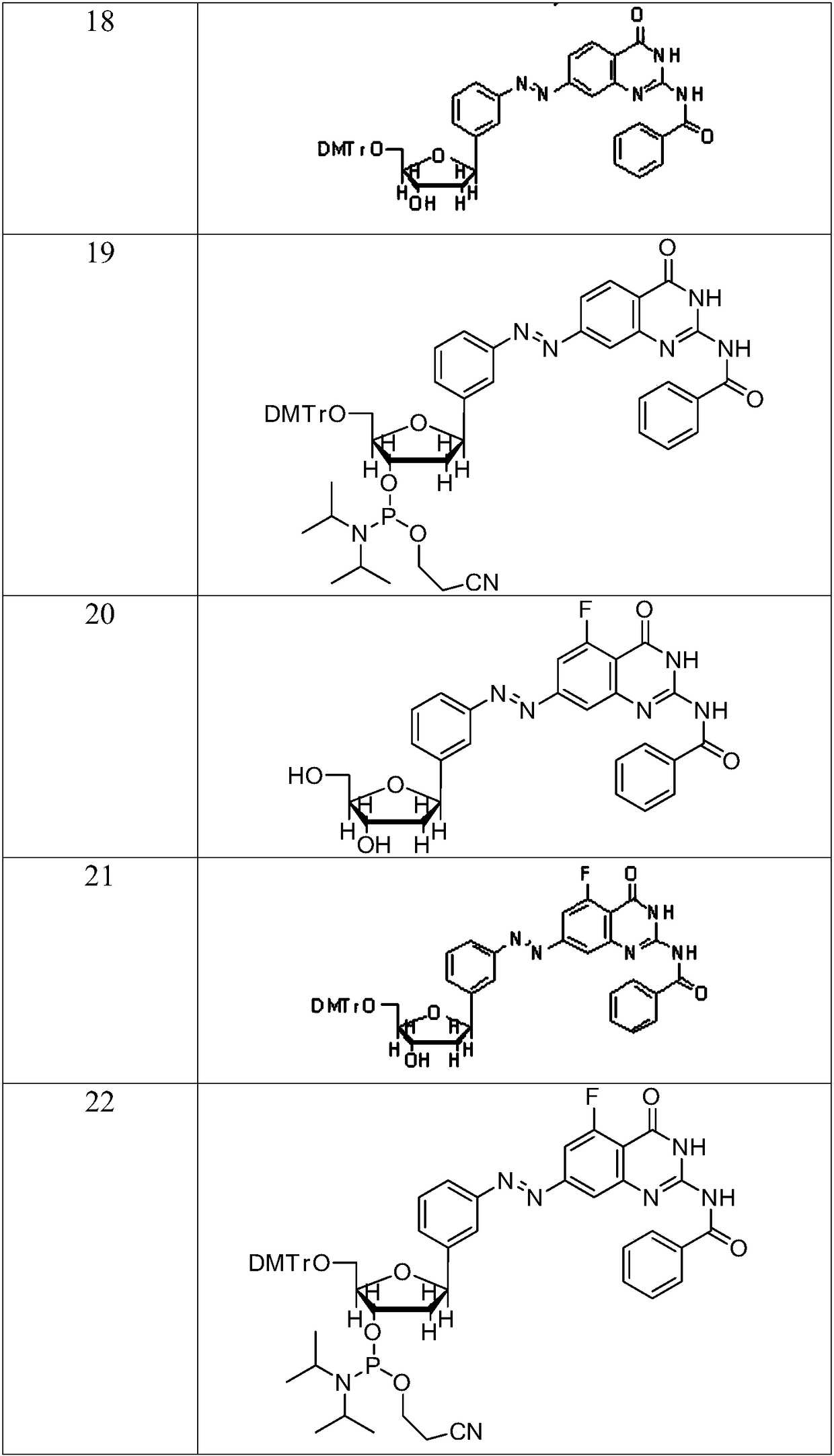
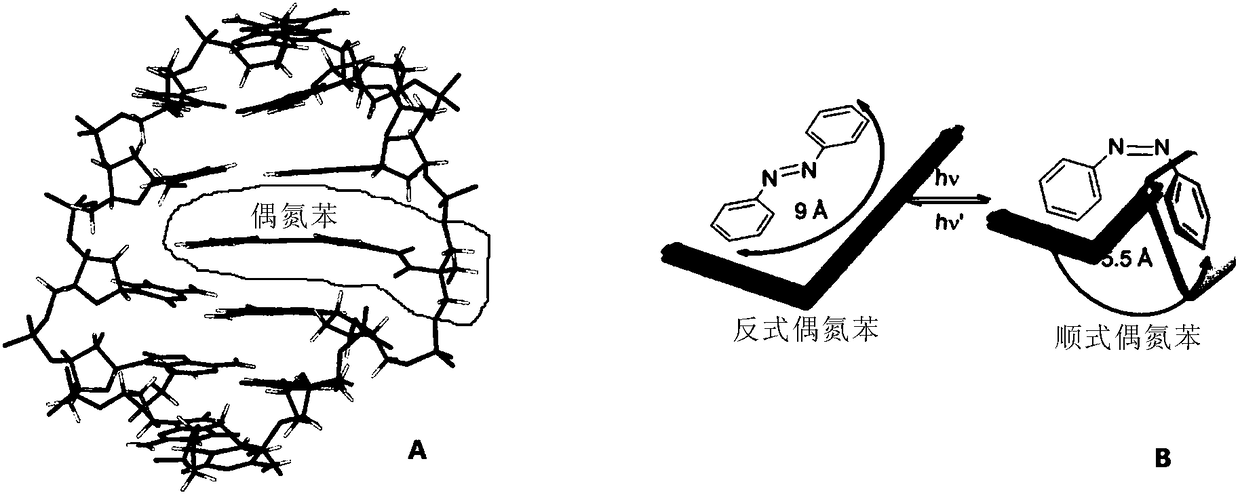
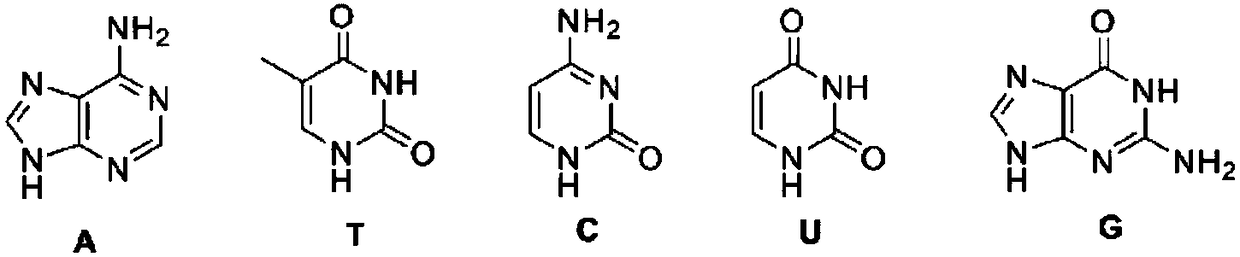
Compound containing azobenzene base element and preparation method and application of compound
A compound, the technology of azophenyl base, which is applied in the field of compounds containing azophenyl base elements and its preparation, can solve the problems of disturbance superposition stability, poor effect, limited effect, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0071] The present invention also provides a preparation method of the compound, comprising the steps of:
[0072] 1) Will and Reaction, obtain described compound;
[0073] And after step 1) also optionally include steps:
[0074] 2) reacting the product obtained in step 1) with 4,4'-bismethoxytrityl chloride to obtain the compound;
[0075] And after step 2) also optionally include steps:
[0076] 3) reacting the product obtained in step 2) with phosphoramidite chloride to obtain the compound,
[0077] in, and R c as defined above.
[0078] application
[0079] The present invention also provides an application of the compound for preparing a composition containing the compound's DNA double-strand or RNA double-strand.
[0080] The present invention also provides a non-diagnostic and non-therapeutic method for regulating the DNA double-strand switch, the DNA double-strand contains the compound, and realizes the control of the reversible switch of the DNA double-stra...
Embodiment 1
[0088] The synthesis of embodiment 1 compound 7
[0089] Synthesis of compound 1:
[0090]
[0091] Synthesis of compound S2 (1,3-bis(4-methylbenzoyl)-7-nitroquinazoline-2,4(1H,3H)-dione):
[0092] In compound S1 (2.07g, 10.0mmol) and triethylamine (Et 3 N, 5ml) was added dropwise to a solution of anhydrous DMF (80mL) at 0 °C (3.56g, 23.0mmol). After the addition, the reaction solution returned to room temperature and continued to stir for 30 minutes. Then the reaction solution was stirred at 50°C for 8 hours, the organic solvent was removed and the residue was dissolved in dichloromethane, washed with brine and dried. The organic phase was removed and the residue was purified by silica gel column chromatography to obtain white solid compound S2 (5.50 g, yield 63%)
[0093] 1 H NMR (300MHz, CDCl 3 )δ8.45(d, J=8.4Hz, 1H), 8.13(dd, J=8.4Hz, J=2.1Hz, 1H), 7.84-7.95(m, 4H), 7.29-7.36(m, 4H), 2.45(s,3H), 2.43(s,3H); 13 C NMR (100MHz, CDCl 3 )δ168.18,166.70,159.68,152.30...
Embodiment 2
[0117] The synthesis of embodiment 2 compound 10
[0118]
[0119] Synthesis of Compound 8:
[0120] Compound 5 (210mg, 1.0mmol) and compound S6 (442mg, 1mmol) were dissolved in anhydrous dry acetic acid (10mL), then the organic solvent was removed, and the residue was purified by column chromatography to obtain red solid compound 8 (473mg , yield 78%).
[0121] 1 H NMR (300MHz, CD 3 OD)δ8.46(d,J=8.6Hz,1H),7.91-7.99(m,6H)7.41-7.75(m,7H),5.14(dd,J=10.5Hz,5.1Hz,1H),4.27( dt, J=5.7,1.5Hz,1H),3.91-3.95(m,1H),3.63-3.65(m,2H),2.48(s,3H), 2.39(s,3H),2.37(s,3H) ,2.17-2.23(m,1H),1.87-1.94(m,1H); 13 C NMR (101MHz, CDCl 3 )δ168.81,167.75,160.98,156.77,152.47,148.47,147.81,147.15,143.96,139.72,130.95,130.67,130.21,130.15,129.93, 129.91,129.23,129.20,129.08,122.25,120.89,118.24,116.44,109.21, 88.18 ,79.71,73.14,62.80,43.70,20.76,20.70; MS(ESI) m / z 633.23 [M+H] + .
[0122] Synthesis of Compound 9:
[0123] DMTrCl (186 mg, 0.55 mmol) was added to a solution of compound 8 (315 m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com