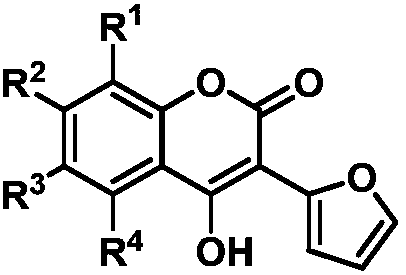
3-(2-furan)-4-hydroxycoumarin compound, preparation method thereof and application of anti-plant fungus
A technology of hydroxycoumarin and compounds, which is applied in the field of 3--4-hydroxycoumarin compounds, can solve the problems of toxicity and pathogenic fungus drug resistance, and achieve short synthesis routes, good plant protection effects, and low production costs low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
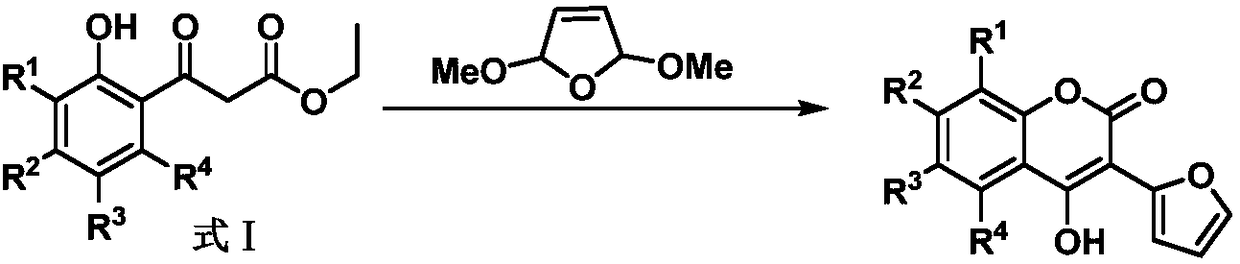
[0023] Preparation of Compounds 3a-3p
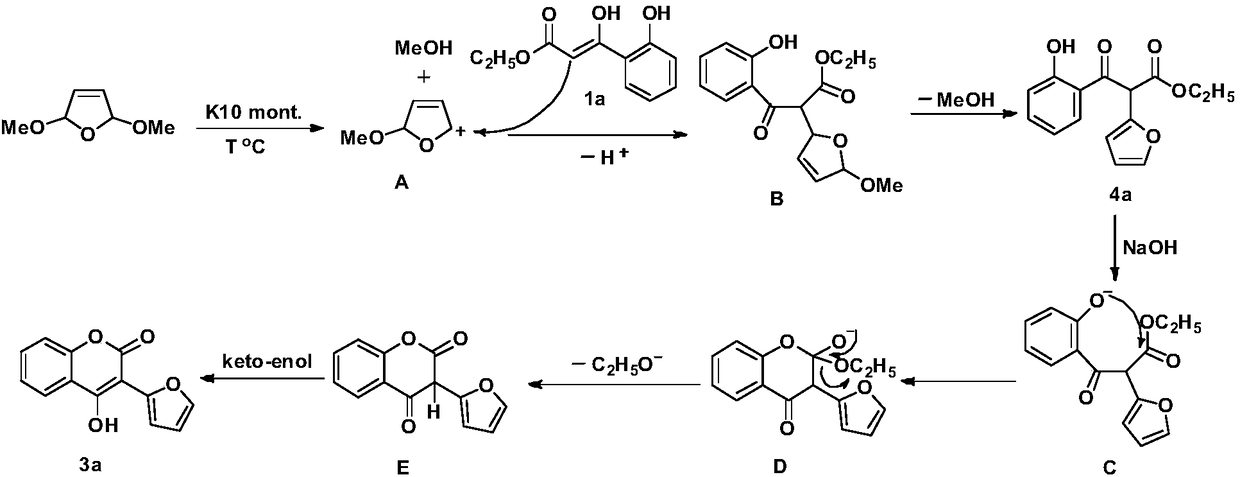
[0024] Add 0.1040g (0.5mmol) ethyl 3-(2-hydroxyphenyl)-3-oxopropionate to the reaction kettle, add 0.1040g K10 montmorillonite under stirring, heat to 80°C, then add 0.0976g ( 0.75mmol) 2,5-dimethoxy-2,5-dihydrofuran, stirred at constant temperature for 1h; then added 0.04g (1mmol) sodium hydroxide, and added 1mL ethanol, refluxed for 0.5h; vacuum K10 montmorillonite was recovered by filtration, and the filtrate was distilled under reduced pressure and purified by column chromatography (eluent: CH 2 Cl 2 ), to obtain the pure product of compound 3a.
[0025] In this example, equimolar 3-(2-hydroxyl-4-methoxyphenyl)-3-oxopropionic acid ethyl ester, 3-(2-hydroxyl-6-methoxyphenyl)-3 -Ethyl oxopropionate, ethyl 3-(2-hydroxy-3-methylphenyl)-3-oxopropionate, ethyl 3-(2-hydroxy-5-methylphenyl)-3-oxopropionate Esters, ethyl 3-(2-hydroxy-4,5-dimethoxyphenyl)-3-oxopropionate, 3-(2-hydroxy-4,6-dimethoxyphenyl)-3 -Ethyl oxopropionate, ethyl 3-(...
Embodiment 2
[0046] In this example, the mass ratio of the amount of K10 montmorillonite to 3-(2-hydroxyphenyl)-3-oxopropionic acid ethyl ester compound is 0.5:1, and other steps and reaction conditions are the same as in Example 1 , Compounds 3a-3p were sequentially obtained, and the product yields are shown in Table 2.
[0047] The productive rate of table 2 compound 3a~3s
[0048] Compound 3a
Embodiment 3
[0050] In this example, the mass ratio of the amount of K10 montmorillonite to 3-(2-hydroxyphenyl)-3-oxopropionic acid ethyl ester compound is 2:1, and other steps and reaction conditions are the same as in Example 1 , Compounds 3a-3p were sequentially obtained, and the product yields are shown in Table 3.
[0051] The productive rate of table 3 compound 3a~3s
[0052] Compound 3a
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com