Purification method of HBcAg (hepatitis B virus core antigen)-VLP or HBcAg-VLP derivative
A purification method and technology of derivatives, which are applied in the field of hydrophobic chromatography purification of HBcAg-VLP or HBcAg-VLP derivatives, can solve the problems of unfavorable large-scale preparation, low economic value, many steps, etc., and achieve great practical application value and Promotion prospect, good repeatability, stable process effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] In this embodiment, the sample solution of HBcAg-VLP is prepared as follows and the pretreatment of the sample solution is carried out
[0066] Plasmid construction and transformation: using the nucleotide sequence of the full-length hepatitis B core antigen, SEQ ID NO.1:
[0067] ATGGACATTGACCCTTATAAAGAATTTGGAGCTACTGTGGAGTTACTCTCGTTTTTGCCTTCTGACTTCTTTCCTTCCGTCAGAGATCTCCTAGACACCGCCTCAGCTCTGTATCGAGAAGCCTTAGAGTCTCCTGAGCATTGCTCACCTCACCATACTGCACTCAGGCAAGCCATTCTCTGCTGGGGGGAATTGATGACTCTAGCTACCTGGGTGGGTAATAATTTGGAAGATCCAGCATCCAGGGATCTAGTAGTCAATTATGTTAATACTAACATGGGTTTAAAGATCAGGCAACTATTGTGGTTTCATATATCTTGCCTTACTTTTGGAAGAGAGACTGTACTTGAATATTTGGTCTCTTTCGGAGTGTGGATTCGCACTCCTCCAGCCTATAGACCACCAAATGCCCCTATCTTATCAACACTTCCGGAAACTACTGTTGTTAGACGACGGGACCGAGGCAGGTCCCCTAGAAGAAGAACTCCCTCGCCTCGCAGACGCAGATCTCAATCGCCGCGTCGCAGAAGATCTCAATCTCGGGAATCTCAATGTTAG.
[0068] Its amino acid sequence SEQ ID NO.2: MDIDPYKEFGATVELLSFLPSDFFFPSVRDLLDTASALYREALESPEHCSPHHTALRQAILCWGELMTLATWVGNNLEDPASRDLVVNYVNTNMGLK...
Embodiment 2
[0077] This example uses ion exchange chromatography to purify HBc-VLP
[0078] The fillers used in this example include conventional DEAE Sepharose FF and Q Sepharose FF anion exchange fillers, and ultra-large pore fillers POROS HQ, POROS 50D and POROS PI.
[0079] In the screening of five kinds of ion-exchange fillers, the supernatant prepared in Example 1 was used as the raw material. After optimizing the pH and elution conditions, the results of yield and final purity were obtained, as shown in Table 1 below. :
[0080] Table 1
[0081]
[0082]
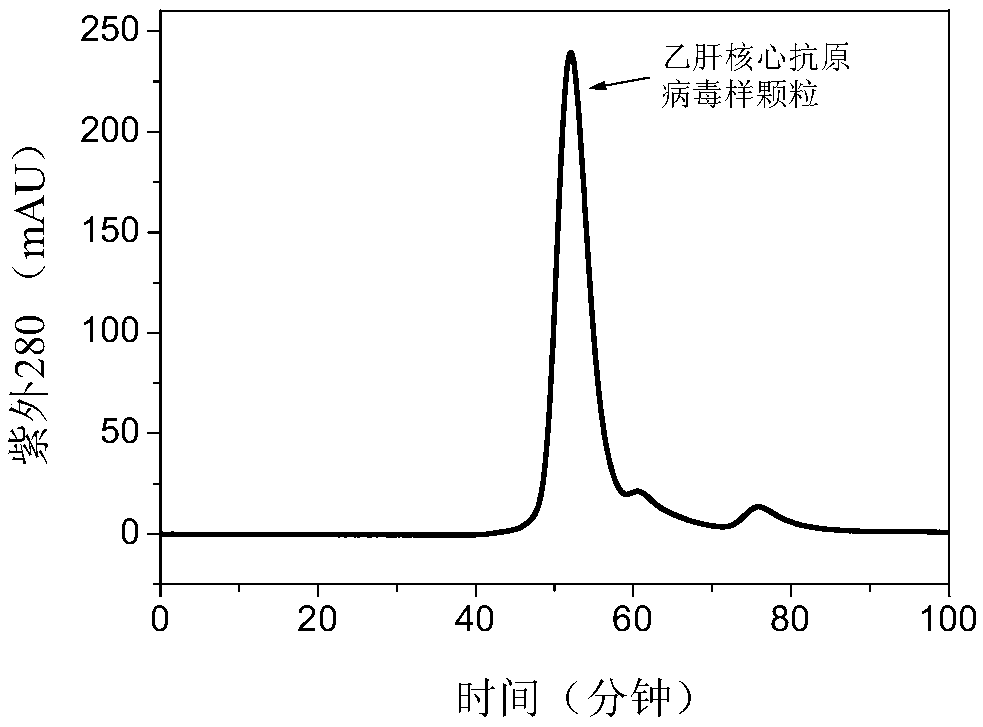
[0083] It can be seen from Table 1 that the best effect of ion exchange chromatography is POROS 50D macroporous anion exchange packing. After purification, the yield of HBc-VLP is 69.64%, and the purity is 37.27%. The effect is equal to or even better than that reported ion-exchange-based purification method.
Embodiment 3
[0085] In this example, the sample solution of HBcAg-VLP was purified using hydrophobic interaction chromatography filler
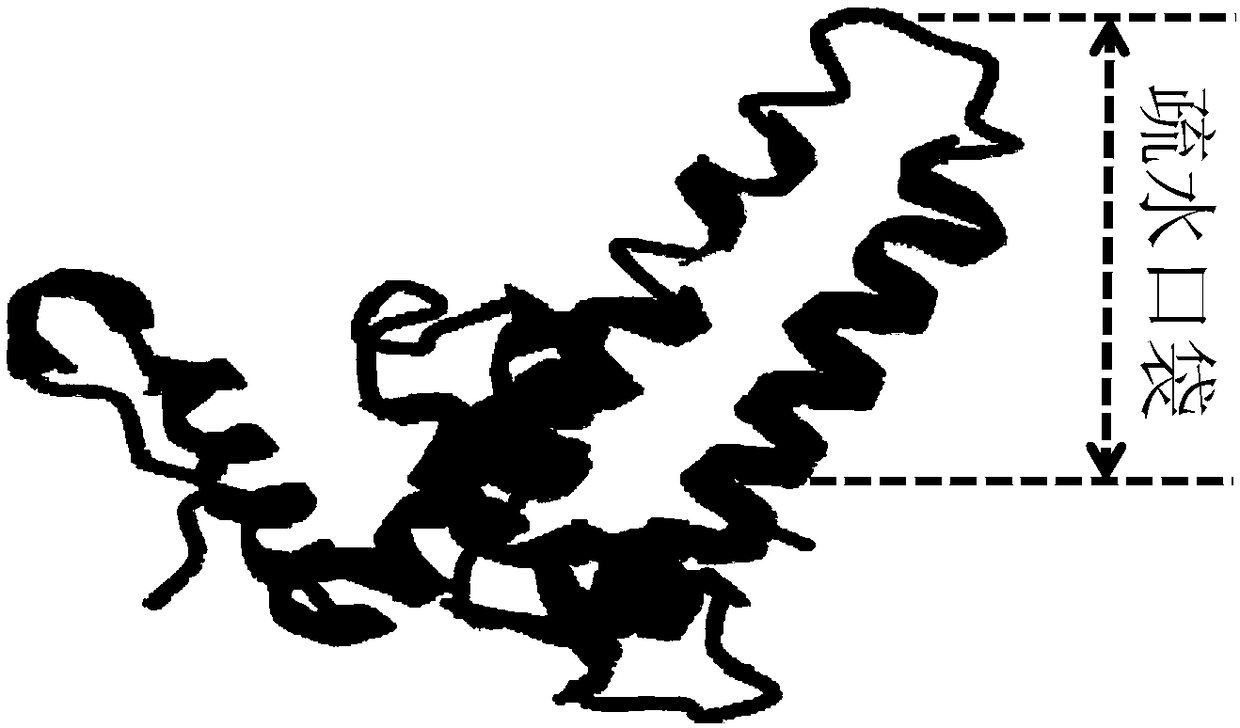
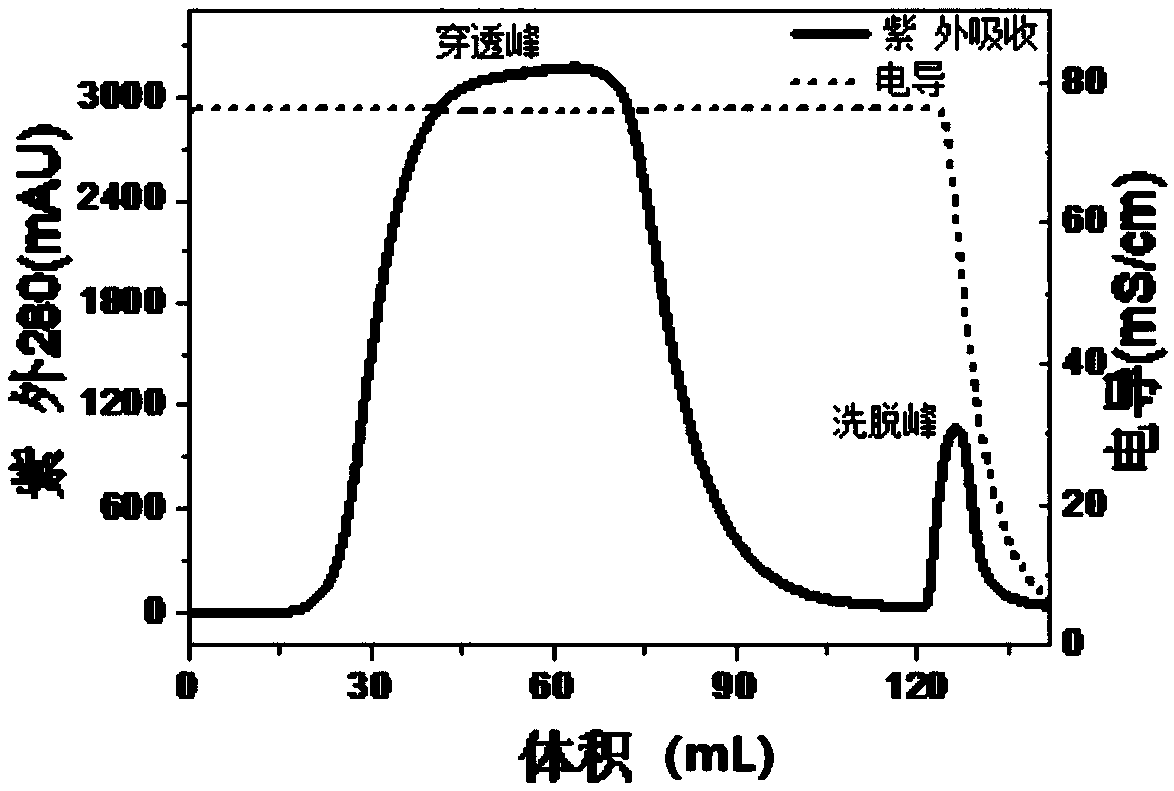
[0086] In view of the presence of strong hydrophobic regions on the surface of HBc-VLP (structural diagram as shown in figure 1 As shown), hydrophobic chromatography mainly relies on the hydrophobic interaction force to achieve the adsorption and desorption of the target protein, which is milder than the ionic interaction force. Therefore, using the sample solution prepared in Example 1 as the raw material, the ligands of the hydrophobic filler mainly include Butyl-S, Butyl, Octyl and Phenyl. In comparison, Butyl-S Sepharose 6FF and Butyl Sepharose 4FF, two relatively weak hydrophobic fillers, are more suitable for the purification of HBc-VLP. After the sample solution was adjusted to a pH of about pH 7.4 with sodium hydroxide solution, it was fed to the hydrophobicity of Butyl-S Sepharose 6FF or Butyl Sepharose 4FF balanced with 20mM phosphate buffer (p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Conductance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com