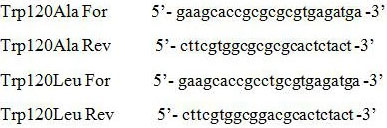A kind of L-amino acid deaminase mutant with improved heat resistance and preparation method thereof
An amino acid and deaminase technology, applied in the biological field, can solve the problems of poor thermal stability, short enzyme life cycle, low enzyme specific activity, etc., and achieve the effects of reducing workload, prolonging service life and improving structural stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Below in conjunction with specific embodiment, further illustrate the present invention.
[0022] The medium formula involved in the embodiment is as follows:
[0023] LB liquid medium: 0.5% yeast extract, 1% peptone, 1% NaCl, pH7.0. Sterilize at 121°C for 20min. When making plates, add 2% agar and sterilize at 121°C for 20min.
[0024] When kanamycin antibiotics were screened, the final concentration was 50mg / L.
[0025] The unit in the above medium is % (W / V).
[0026] Example 1:
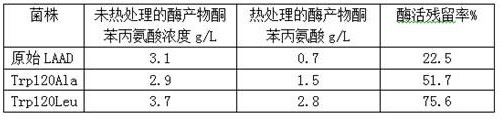
[0027] 1. Construction of 120 amino acid site mutants of L-amino acid deaminase
[0028] Total gene synthesis from Proteus mirabilis ( Proteus mirabilis ) L-amino acid deaminase (L-aminoacid deaminase, LAAD) gene (genbank accession number EU669819.1), Nde I and Hind Ⅲ Connected into pET24a, constructed plasmid LAAD, transformed into E. coli BL21(DE3), the recombinant strain PMLAAD was constructed.
[0029] The plasmid LAAD of the recombinant strain PMLAAD was extracted, and the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com