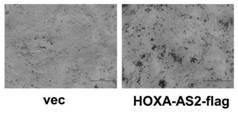
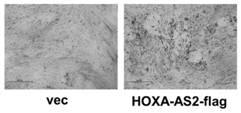
Application of long chain non-coded RNA-HOXA-AS2 in bone tissue damage repair
An RNA-HOXA-AS2, HOXA-AS2 technology, applied in the application field of long-chain non-coding RNA-HOXA-AS2 in the repair of bone tissue damage, can solve the problems of immune rejection, many complications, few sources, etc. The effect of enhancing alkaline phosphatase activity and promoting calcium ion deposition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: Acquisition of HOXA-AS2 gene fragments
[0031] According to the RNA nucleotide sequence (RefSeq sequence number: NR_122069.1, as shown in SEQ ID NO: 1 in the sequence listing) of the long-chain non-coding RNA-HOXA-AS2 in the NCBI database and BamH I on the lentiviral vector pLV and Xba I-specific enzyme cutting site, using DNA chem192 synthesizer, according to the principle of solid-phase phosphoramidite triester method, double-strand synthesis of HOXA-AS2 gene fragment, and adding two Specific enzyme cutting sites BamH I (GGATCC) and Xba I (TCTAGA) were obtained to obtain a HOXA-AS2 gene fragment containing specific enzyme cutting sites, the nucleotide sequence of which is shown in SEQ ID NO: 2 in the sequence listing.
Embodiment 2
[0032] Example 2: Construction of lentiviral expression vector pLV-HOXA-AS2
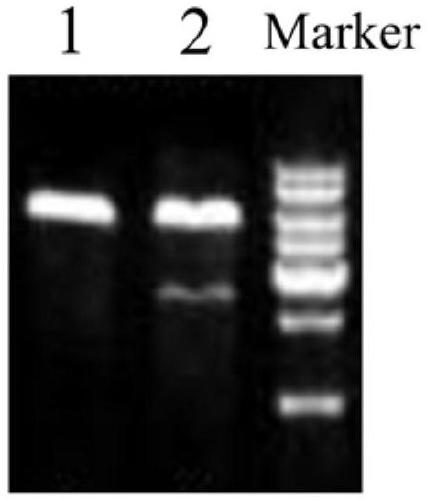
[0033] Using restriction endonucleases BamH I and Xba I, the lentiviral empty vector pLV (its nucleotide sequence is shown in SEQ ID NO: 3 in the sequence listing) and the HOXA-AS2 gene fragment obtained in Example 1 were respectively carried out Double digestion, use T4 DNA ligase system to ligate the digested HOXA-AS2 gene fragment and linear lentiviral empty vector pLV, then transform competent cells, screen positive colonies, extract plasmids of positive colonies, and obtain lentiviral expression Vector pLV-HOXA-AS2.
[0034] 1. Enzyme digestion of lentiviral empty vector pLV
[0035]The enzyme digestion reaction system is as follows (20 μL):
[0036]
[0037] Reaction conditions for enzyme cleavage: react at 37°C for 4 hours.
[0038] 2. Enzyme digestion of HOXA-AS2 gene fragment
[0039] The enzyme digestion reaction system is as follows (20 μL):
[0040]
[0041] Reaction conditions...
Embodiment 3
[0054] Example 3: Lentiviral Packaging
[0055] HEK293T cells were seeded in six-well culture plates for culture, and cultured in a cell culture incubator at 37°C with DMEM complete medium containing 10% fetal bovine serum (FBS). When the cell density reached about 70%, the Lipofectamine 3000 liposome transfection reagent (purchased from Thermo Fisher, model L3000015), respectively transfected lentiviral expression vector pLV-HOXA-AS2 and lentiviral empty vector pLV into HEK293T cells for lentiviral packaging. Specific steps are as follows:
[0056] 1) Take two 1.5mL EP tubes and add 150μL serum-free DMEM medium to each;
[0057] 2) Add 1 μg lentiviral expression vector pLV-HOXA-AS2, 0.5 μg lentiviral packaging plasmid pSPAX2, and 0.5 μg lentiviral packaging plasmid pMD2G into one of the EP tubes, and mix well;
[0058] 3) Add Lipofectamine 3000 liposome transfection reagent into another EP tube and mix well;
[0059] 4) Quickly add the plasmid solution obtained in step 2) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com