Method for biologically synthesizing glutaric acid
A biosynthesis, glutaric acid technology, applied in the biological field, can solve the problem that intermediate products cannot be used again
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment 1
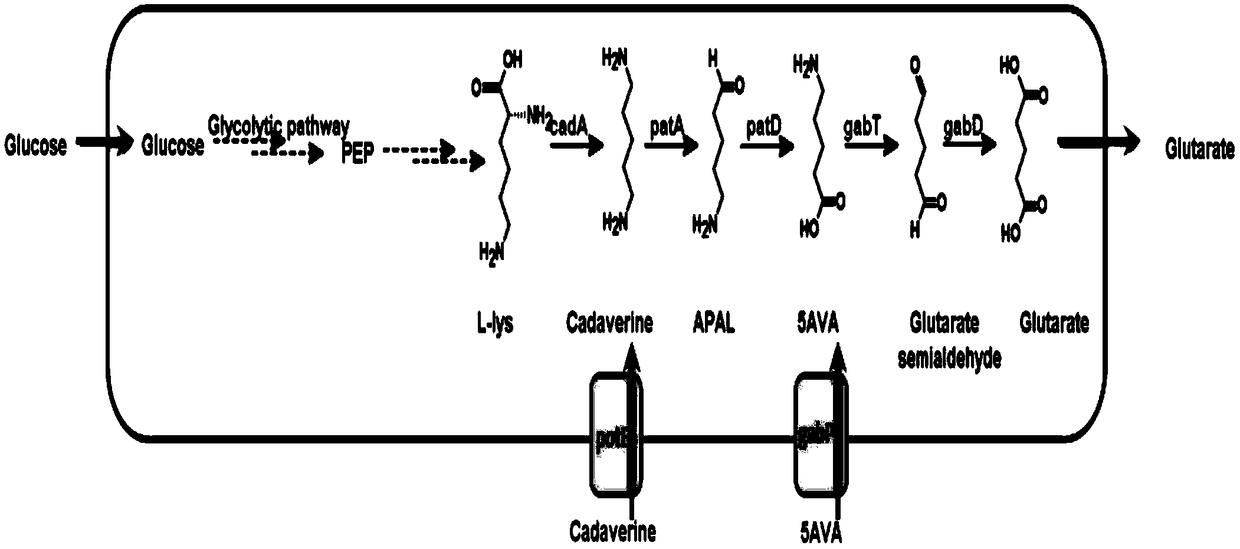
[0019] Modular optimization of glutaric acid production strains
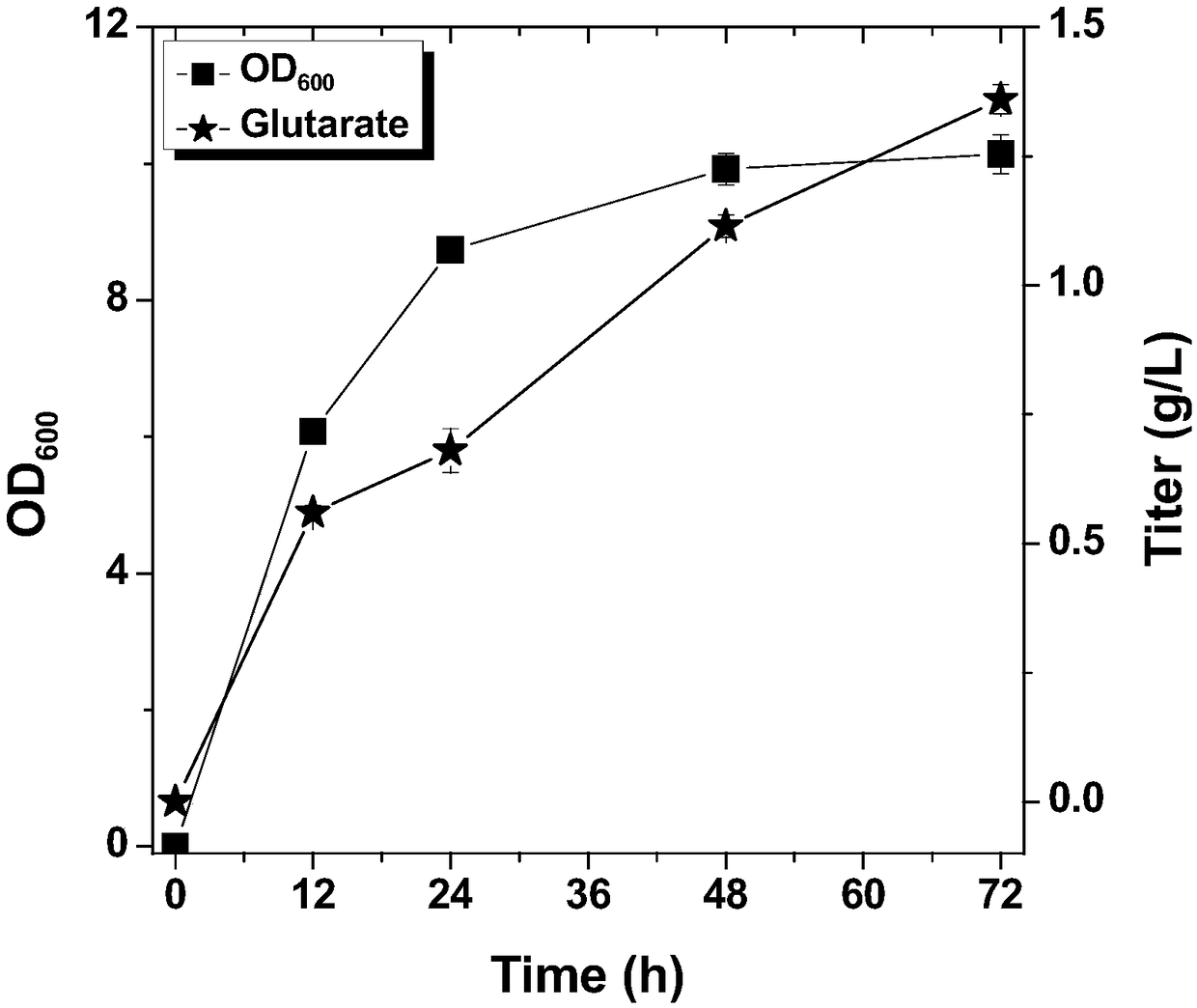
[0020] Select the lysine decarboxylase cadA, pentane diamine aminotransferase patA, 5-aminovalerate semialdehyde dehydrogenase patD, 5-aminovalerate aminotransferase gabT, and glutaric acid semialdehyde dehydrogenase gabD from E. coli, obtained by PCR Gene fragment, then double-enzyme digestion of the fragment and vector with endonuclease, the digested fragment is recovered by gel or column, and then the target gene is inserted into the plasmid pZE12-luc (high copy), pCS27 (medium Copy), pSA74 (low copy). Regulate the expression of genes, detect the accumulation of target products and intermediate products, and optimize the host strain to obtain pSA-cadA, pCS-patAD-gabTD recombinant plasmids (Table 1).
[0021] Prepare competent cells by electroporation, and aliquot 100 μL into 1.5 mL EP tubes for transformation. Add 2 μL of the constructed recombinant plasmid pSA-cadA and 2 μL of pCS-patAD-gabTD into a 1.5 mL cent...
specific Embodiment 2
[0024] Co-expression of pentanediamine transporter potE and 5-aminovalerate transporter gabP accelerate glutaric acid synthesis
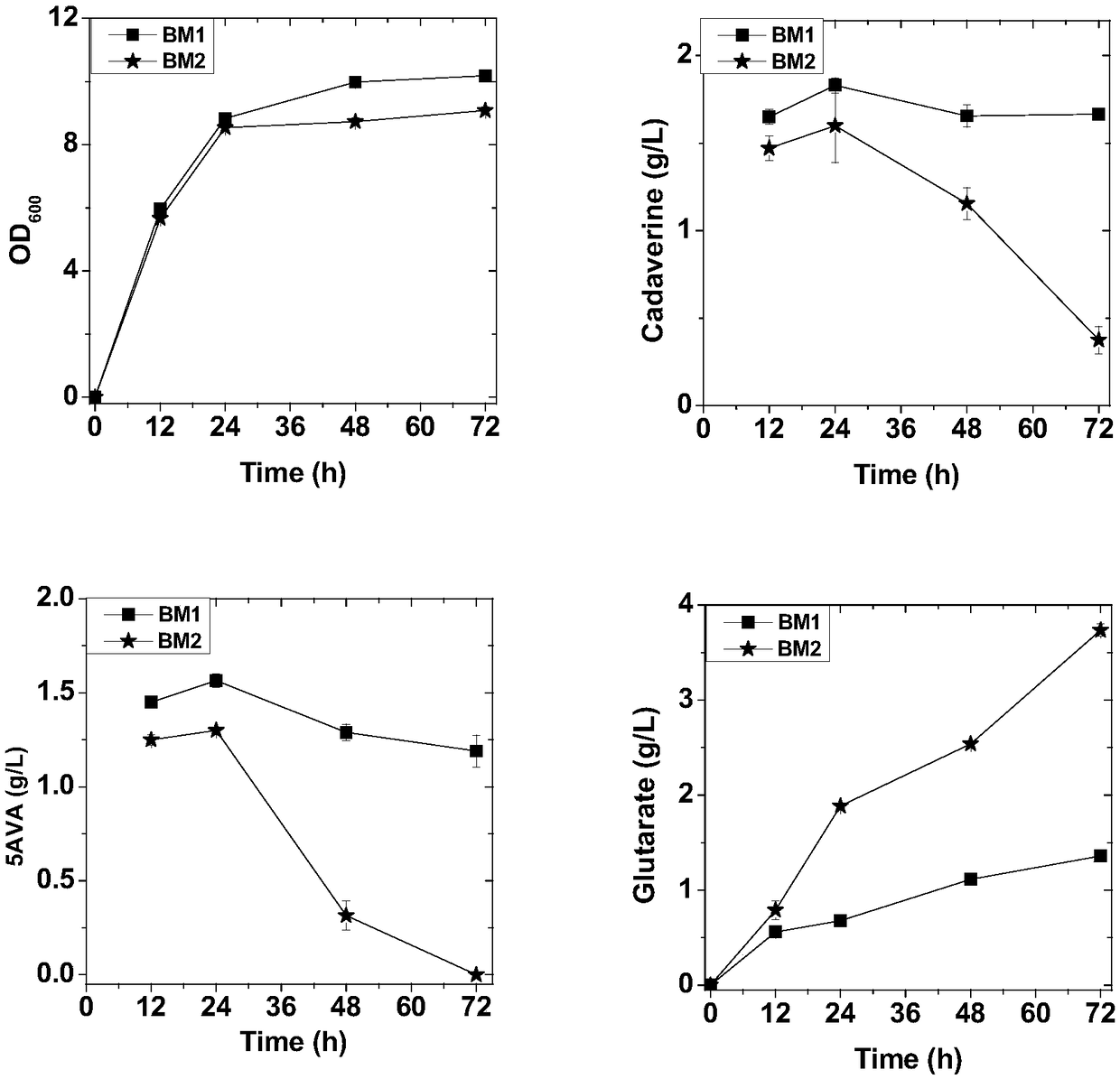
[0025] The potE, 5-aminovalerate transporter gabP from E. coli was selected, the fragments were obtained by PCR, and then the fragments and vectors were digested with endonuclease, and the digested fragments were recovered by gel or column, and then the target The genes potE and gabP were inserted into the plasmids pSA-cadA and pCS-patAD-gabTD to obtain pSA-cadA-potE and pCS-patAD-gabTDP recombinant plasmids (Table 1).
[0026] Prepare competent cells by electroporation, and aliquot 100 μL into 1.5 mL EP tubes for transformation. Add 2μL each of the constructed recombinant plasmids pSA-cadA-potE and pCS-patAD-gabTDP into a 1.5mL centrifuge tube containing 100μL of competent cells, and mix well. Then use an electroporator to electrotransform the plasmid into competent cells. After the electroporation is completed, add LB medium, and transfer the mixture...
specific Embodiment 3
[0029] Construction of metabolic pathway for de novo synthesis of glutaric acid by engineered strains
[0030] After the biotransformation of lysine into glutaric acid is achieved, the enzymes lysA, dapB, and lysC are used to enhance the upstream lysine flux fbr The expression of can realize the de novo synthesis of glutaric acid. The gene fragments were obtained by PCR, and then the fragments and the vector were digested with endonuclease. The digested fragments were recovered by gel or column, and then the target gene was inserted into the plasmid pZE12-luc to obtain pZE-lysA- dapB-lysC fbr (Table 1).
[0031] Prepare competent cells by electroporation, and aliquot 100μL into 1.5mL EP tubes for transformation. The constructed recombinant plasmid pZE-lysA-dapB-lysC fbr 2μL was added to a 1.5mL centrifuge tube containing 100μL of competent cells, and mixed well. Then use an electroporator to electrotransform the plasmid into competent cells. After the electroporation is c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com