Fluorescence probe for quickly identifying thiophenol
A fluorescent probe and thiophenol technology, which is applied in the field of chemical analysis, can solve the problems of poor water solubility of fluorescent probes, insufficient response speed, and weak anti-interference ability, and achieves strong anti-interference ability, rapid response, and low response speed. quick effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] A method for preparing a fluorescent probe for rapid detection of thiophenol, specifically comprising the following steps:
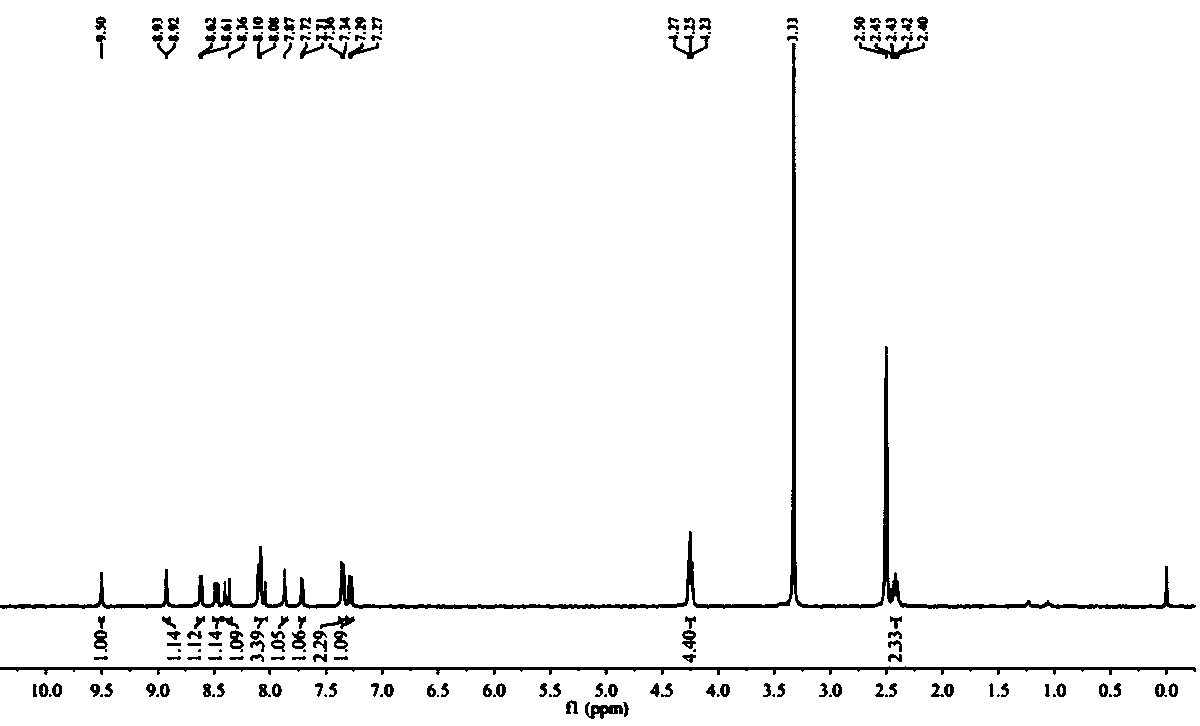
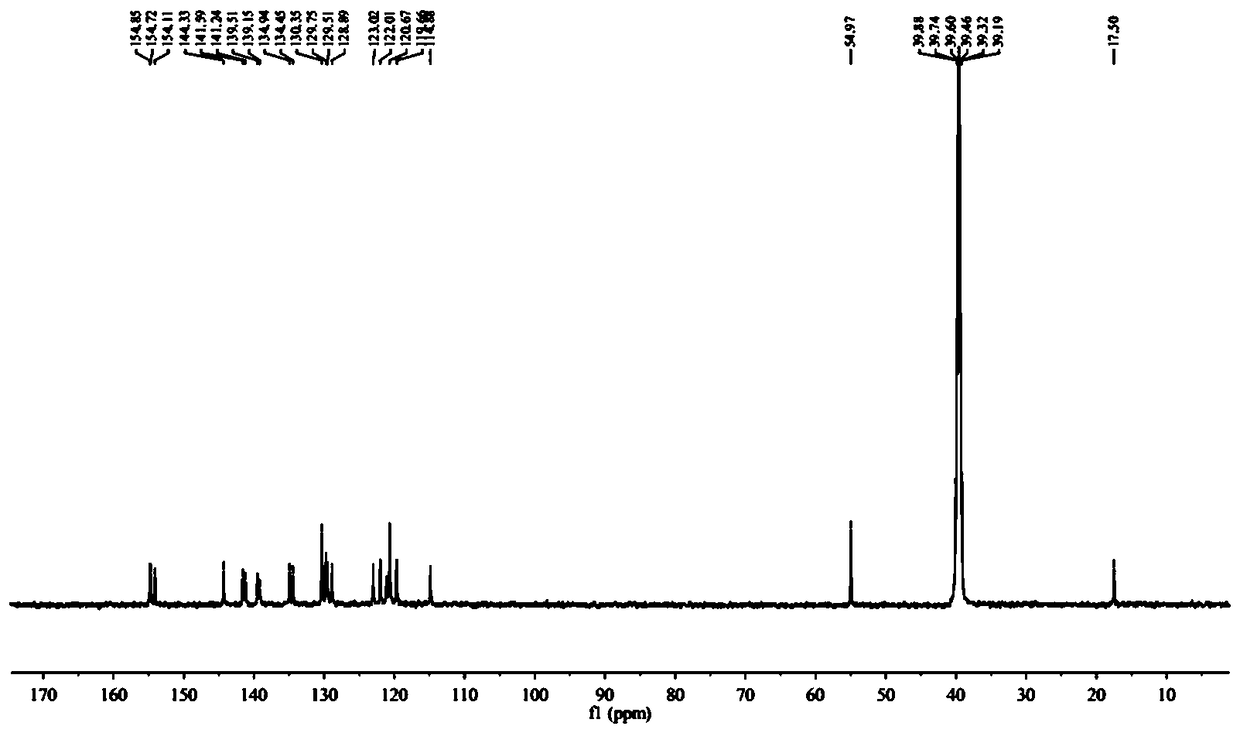
[0038] Synthesis of Intermediate A: Dissolve 1 g (6.84 mmol) of 2,7-naphthyridin-1(2H)-one and 9.81 g (34.21 mmol) of phosphorus oxybromide in 10 mL of acetonitrile and heat to 80 ℃ for 1 h. Add ice water to the reaction system to quench the phosphorus oxybromide, then adjust the pH to neutral. The solution was extracted 3 times with dichloromethane, then with Na 2 SO 4 The dichloromethane extract was dried and distilled under reduced pressure to obtain a red crude product, which was further purified by silica gel column chromatography to obtain a white solid, namely Intermediate A (980 mg, yield 69%). 1H NMR (400 MHz, CDC13): δ 9.76 (s, 1H), 8.82 (d, J = 4 Hz, 1H),8.48 (d, J = 4 Hz, 1H), 7.68 (d, J = 8 Hz, 1H), 7.61 (d, J = 8 Hz, 1H).
[0039] Synthesis of Intermediate B: Under argon, 400 mg (1.91 mmol) of Intermediate A and 134 mg (0.19 mmo...
Embodiment 2
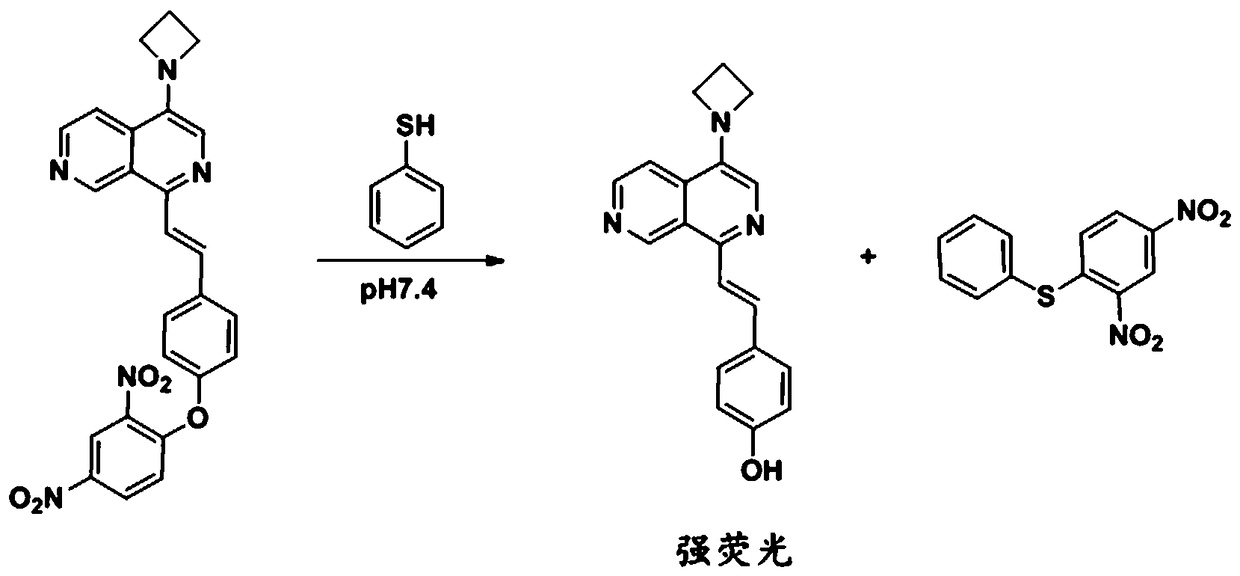
[0046] The effect of the fluorescent probe obtained in Example 1 reacting with thiophenol was determined.
[0047] A 100 μM fluorescent probe solution in Example 1 was prepared using 10 mM PBS (pH=7.4) buffer solution, and a 10 mM thiophenol stock solution was prepared with acetonitrile. Take 100 μL of the above-mentioned fluorescent probe solution and 900 μL PBS (pH=7.4) buffer solution in a cuvette, and detect its fluorescence spectrum under 355 nm excitation, as shown in Figure 4 The solid line part; take 100 μL of the above compound f solution, 895 μL of PBS (pH=7.4) buffer solution and 5 μL of thiophenol stock solution in a cuvette, and detect its fluorescence spectrum under 355 nm excitation, as shown in Figure 4 imaginary part.
[0048] from Figure 4 Comparing the dotted line and the solid line, it can be seen that the background of the fluorescent probe is low and the response to thiophenol is strong, which is a fluorescence-enhanced thiophenol probe.
Embodiment 3
[0050] The analysis of the reaction of the fluorescent probe obtained in Example 1 with different concentrations of thiophenol was determined.
[0051] Add 100 μL of fluorescent probe solution and PBS (pH=7.4) buffer solution to the cuvette, and then add working concentrations of 0, 2, 4, 6, 8, 10, 20, 40, 60, 80, 90 , 100 μM thiophenol was prepared into a 1 mL reaction system, and the fluorescence spectra of different concentrations of thiophenol reacted with the fluorescent probe for 30 seconds were measured, as shown in Figure 5 shown.
[0052] from Figure 5 It can be seen that within a certain concentration range, the fluorescence intensity of the reaction system at 580 nm increases with the concentration of thiophenol, indicating that the probe can detect thiophenol at different concentrations.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com