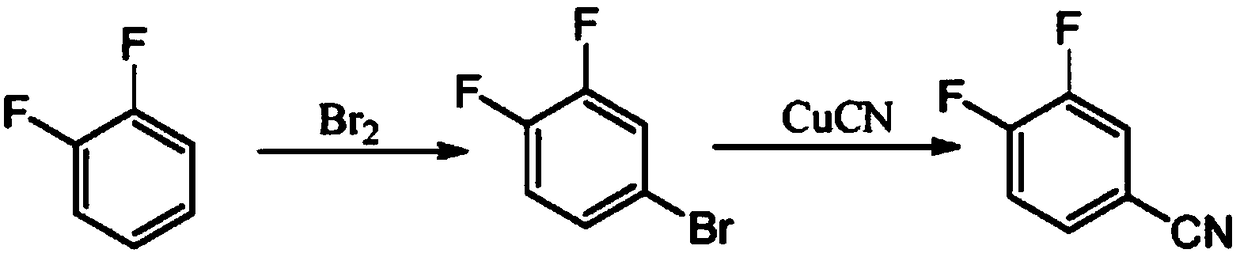
Method for preparing 3,4-difluorobenzonitrile
A technology for difluorobenzonitrile and difluorotoluene is applied in the field of preparing 3,4-difluorobenzonitrile, which can solve the problems of complicated post-processing, influence, long reaction time, etc., and achieves improved purity, simple preparation process and low cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
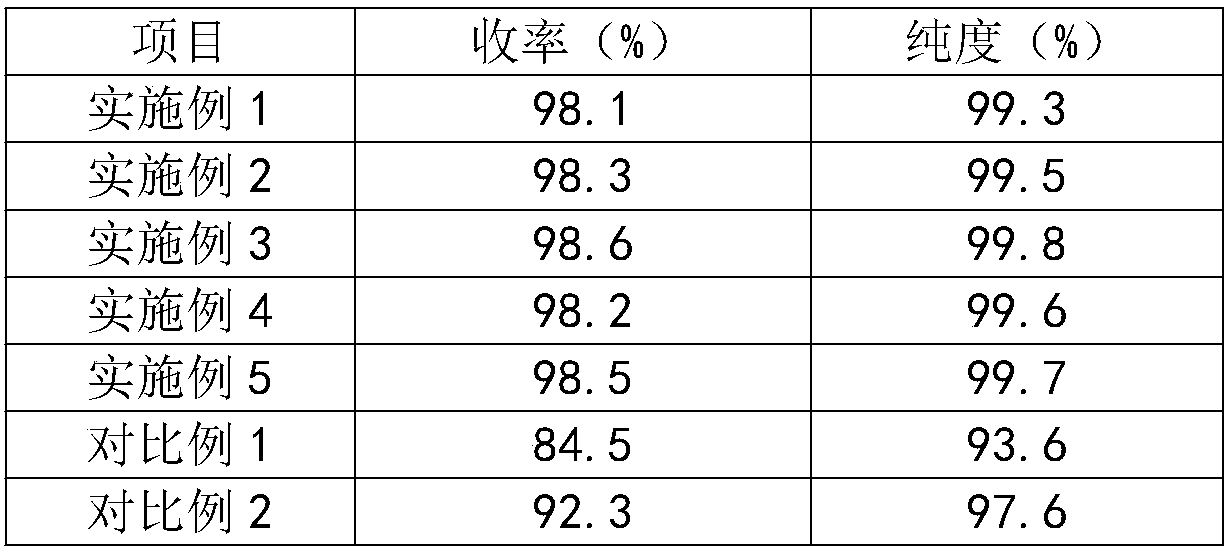
Embodiment 1
[0031] The method for preparing 3,4-difluorobenzonitrile of the present embodiment comprises the following steps:
[0032] (1) Add 3,4-difluorotoluene and antimony pentachloride into the chlorination reaction kettle, turn on the light system, and heat up to 120°C, start to pass chlorine gas, and control the speed of chlorine flow, and at the same time, the The HCl gas is extracted under negative pressure. When the chlorination reaction is over, the chlorine gas is stopped and the heat preservation is continued for 1 hour;
[0033] (2) put the product of step (1) in the hydrolysis reaction kettle, steam heating, when the material temperature reaches 110 ℃, add zinc chloride and polar aprotic solvent, carry out hydrolysis reaction under the condition of rising temperature and reflux, then place In the desolvation kettle, under the vacuum degree of 0.06Mpa, pass steam and heat to 130°C to fractionate the polar aprotic solvent, and then obtain fluorinated benzaldehyde crystals aft...
Embodiment 2
[0047] The method for preparing 3,4-difluorobenzonitrile of the present embodiment comprises the following steps:
[0048] (1) Add 3,4-difluorotoluene and antimony pentachloride into the chlorination reaction kettle, turn on the light system, and heat up to 130°C, start to pass chlorine gas, and control the speed of chlorine flow, and at the same time, the The HCl gas is extracted under negative pressure. When the chlorination reaction is over, the chlorine gas is stopped and the heat preservation is continued for 2 hours;
[0049] (2) put the product of step (1) in the hydrolysis reaction kettle, steam heating, when the material temperature reaches 120 ℃, add zinc chloride and polar aprotic solvent, carry out hydrolysis reaction under the condition of reflux of raising temperature, then place In the desolvation kettle, under the vacuum degree of 0.1Mpa, pass steam and heat to 140°C to fractionate the polar aprotic solvent, and then obtain fluorinated benzaldehyde crystals aft...
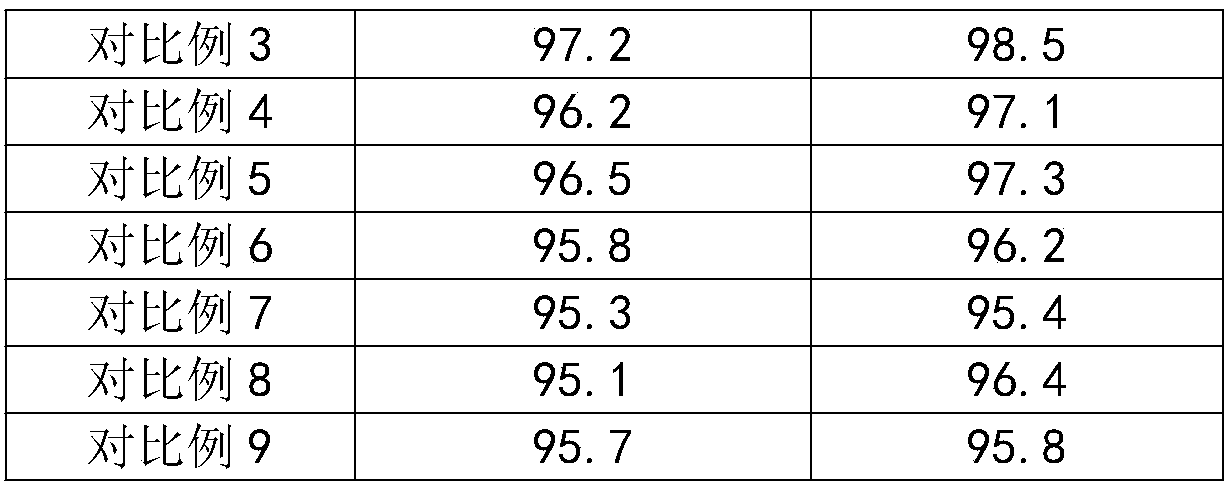
Embodiment 3
[0063] The method for preparing 3,4-difluorobenzonitrile of the present embodiment comprises the following steps:
[0064] (1) Add 3,4-difluorotoluene and antimony pentachloride into the chlorination reaction kettle, turn on the light system, heat up to 15°C, start to pass chlorine gas, and control the speed of chlorine flow, and at the same time, The HCl gas is extracted under negative pressure. When the chlorination reaction is over, the chlorine gas is stopped and the heat preservation is continued for 1.5h;
[0065] (2) put the product of step (1) in the hydrolysis reaction kettle, steam heating, when the material temperature reaches 115 ℃, add zinc chloride and polar aprotic solvent, carry out the hydrolysis reaction under the reflux condition of raising temperature, then place In the desolvation kettle, under the vacuum degree of 0.08Mpa, pass steam and heat to 135°C to fractionate the polar aprotic solvent, and then obtain fluorinated benzaldehyde crystals after layerin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com