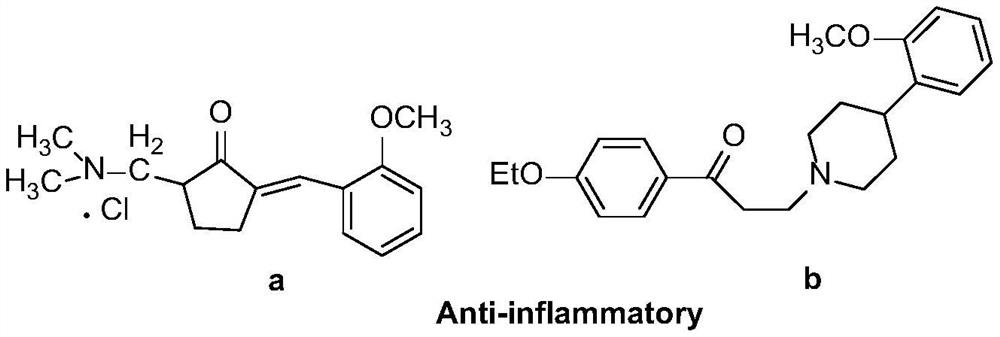
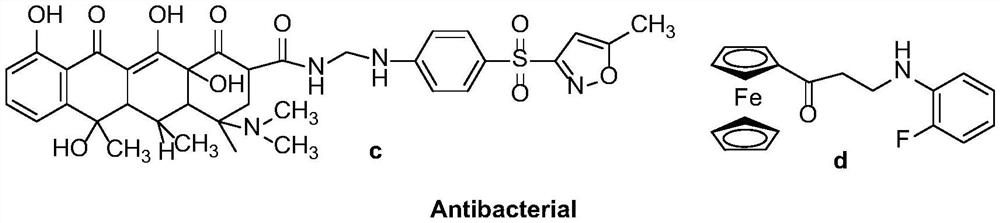
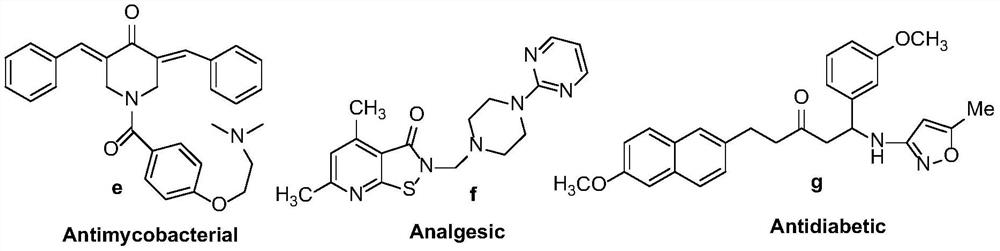
Synthetic method of a class of optically active β-amino ketones
A synthetic method and optically active technology, applied in the field of β-aminoketone compound synthesis, can solve the problems of difficult selection of enzymes or strains, complicated post-processing, difficult control of conditions, etc., and achieve high enantioselectivity excess value, atomic High utilization rate and good substrate adaptability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Preparation of 3-(2-fluorophenyl)-3-((6-methoxybenzo[d]thiazole-2-amino)-1-phenyl-1-propanone (compound number is a):
[0048] At room temperature, add 14.3mg (0.05mmol) of 2-fluorophenyl-6-methoxybenzothiazolimine to a 10mL single-necked bottle, and weigh 3.15mg (10mol%) of 3,5-bistrifluoromethane Add the benzyl bromide quinine quaternary ammonium salt catalyst, add 1.5mL of toluene at room temperature and stir for 5min until the solids are completely dissolved, add 1.5 times the reaction equivalent of 10% KOH aqueous solution, move the reaction system into a cold bath, stir for 10-20mins, Then weigh 9.00mg (0.075mmol) of acetophenone dissolved in 1.0mL of toluene and slowly add it dropwise into the reaction system, continue to stir the reaction at -20°C, TLC plate detection, stop the reaction after 48h of reaction, the initial product of the reaction was subjected to Flash chromatography Column (Biotage Isolera One (Biotage, medium and low pressure preparative liquid ...
Embodiment 2
[0051] Preparation of 1,3-bis(2-fluorophenyl)-3-((6-methoxybenzo[d]thiazole-2-amino)-1-propanone (compound number is b):
[0052] Prepared according to the method and conditions of Example 1, except that 10.35 mg (0.075 mmol) of 2'-fluoroacetophenone was added to obtain 17.2 mg of the target compound with a yield of 81% and an enantiomer selection value of 94% ee.
Embodiment 3
[0054] 1-(2-chlorophenyl)-3-(2-fluorophenyl)-3-((6-methoxybenzo[d]thiazole-2-amino)-1-propanone (compound number is c) preparation:
[0055] Prepared according to the method and conditions of Example 1, except that 11.55 mg (0.075 mmol) of 2'-chloroacetophenone was added to obtain 18.5 mg of the target compound, with a yield of 84%. Enantiomeric selection value: 94% ee.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com