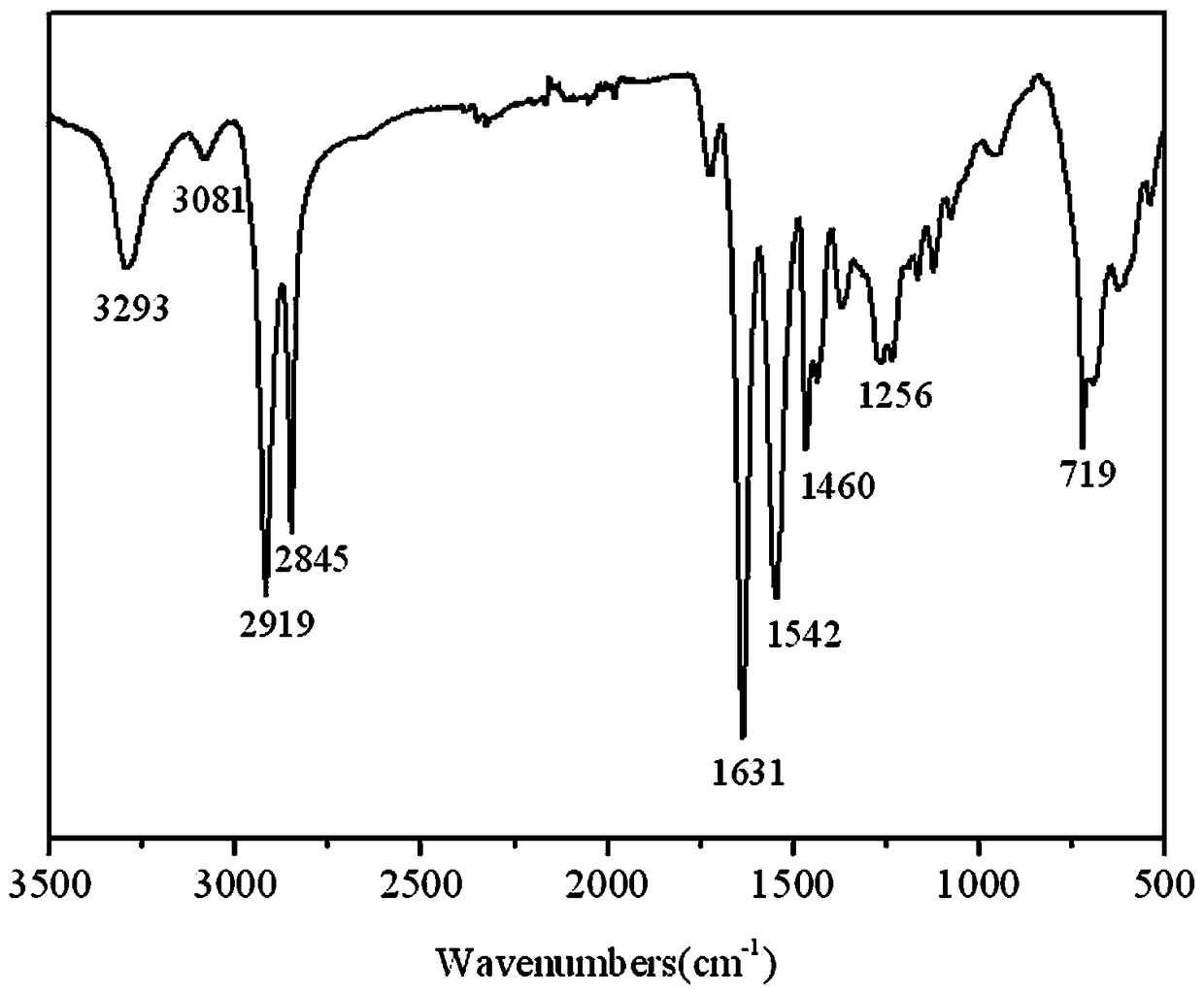
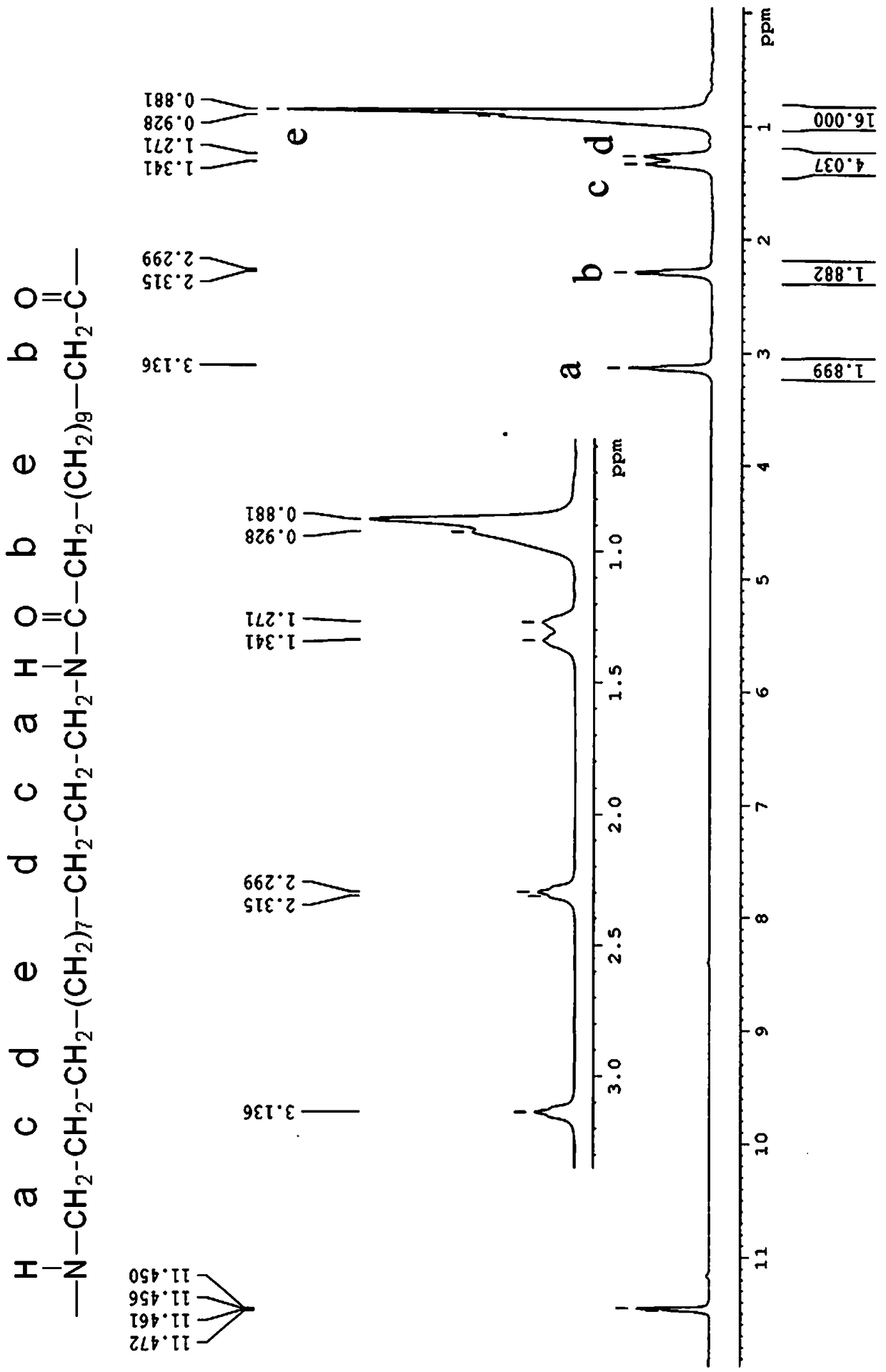
Preparation method for long carbon chain nylon PA1313 and product
A long carbon chain, nylon technology, applied in the field of polymer material synthesis, can solve problems such as low solubility and inability to form salt, and achieve the effects of low recovery cost, reduced equipment investment, and low requirements for equipment safety grades
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] A preparation method of long carbon chain nylon PA1313, comprising the following steps:
[0034] (1) Preparation of PA1313 nylon salt: Add 1,13-tridecanediamine to water with 5 times the mass number, heat to 80°C, and then follow the steps of 1,13-tridecanedibasic acid and 1,13- The molar ratio of thirteen carbon diamine is 1:1.1, add the metered 1,13-tridecanyl diamine, heat to 130°C in an inert gas atmosphere (nitrogen), keep the pressure in the kettle at 0.5MPa, measure the pH of the system According to the measured pH value, add an appropriate amount of 1,13-tridecanedibasic acid or 1,13-tridecanediamine, adjust the pH value of the system at 7.2, and continue the reaction for 25 minutes to obtain PA1313 salt. After centrifugation and dehydration, the PA1313 salt with a water content of 20 wt% was obtained;
[0035] (2) Polymerization of PA1313: Add PA1313 salt with a water content of 20wt% into the polymerization kettle, replace the gas in the kettle with nitrogen ...
Embodiment 2
[0043] A preparation method of long carbon chain nylon PA1313, comprising the following steps:
[0044] (1) Preparation of PA1313 nylon salt: Add 1,13-tridecanediamine to water with 3 times the mass number, heat to 60°C, and then follow the steps of 1,13-tridecanedibasic acid and 1,13- The molar ratio of tridecanyl diamine is 1:1.2. Add the metered 1,13-tridecyl diamine, heat to 120°C in an inert gas atmosphere, keep the pressure in the kettle at 0.4MPa, measure the pH value of the system, according to Add an appropriate amount of 1,13-tridecanedibasic acid or 1,13-tridecanediamine to the measured pH value, adjust the pH value of the system to 7.4, and continue the reaction for 30 minutes to obtain PA1313 salt. After centrifugation and dehydration Obtaining water content is the PA1313 salt of 15wt%;
[0045] (2) Polymerization of PA1313: Add PA1313 salt with a water content of 15wt% into the polymerization kettle, replace the gas in the kettle with inert gas nitrogen to ensur...
Embodiment 3
[0053] A preparation method of long carbon chain nylon PA1313, comprising the following steps:
[0054] 1) Preparation of PA1313 nylon salt: add 1,13-tridecanediamine to water with 8 times the mass number, heat to 80°C, and then follow the steps of 1,13-tridecanedioic acid and 1,13-deca The molar ratio of three-carbon diamine is 1:1.05. Add metered 1,13-tridecyl diamine, heat to 180°C in an inert gas atmosphere, keep the pressure in the kettle at 1.2MPa, and measure the pH value of the system. Add an appropriate amount of 1,13-tridecanedibasic acid or 1,13-tridecanediamine to adjust the pH of the system to 7.1, and continue the reaction for 20 minutes to obtain PA1313 salt, which is obtained after centrifugation and dehydration PA1313 salt with a water content of 30% by weight;
[0055](2) Polymerization of PA1313: Add PA1313 salt with a water content of 30wt% into the polymerization kettle, and replace the gas in the kettle with nitrogen gas to ensure that the reaction kettl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
| heat deflection temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com