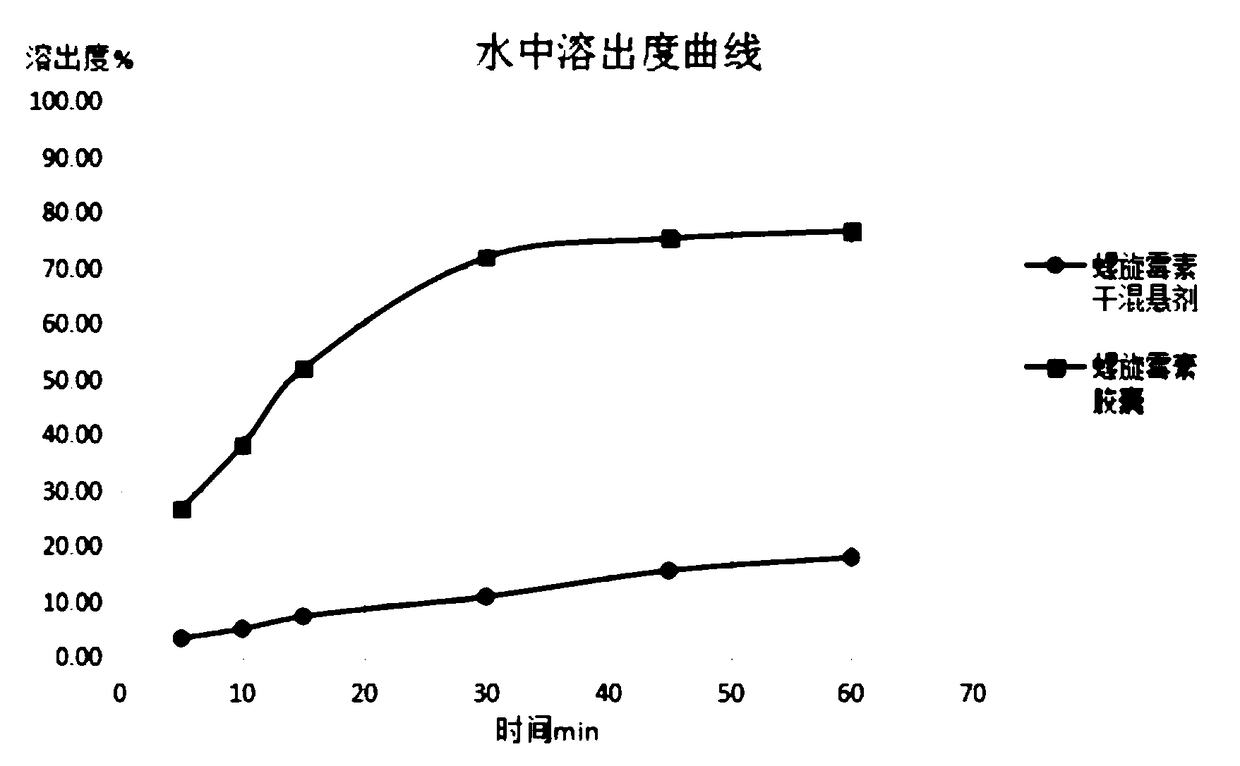
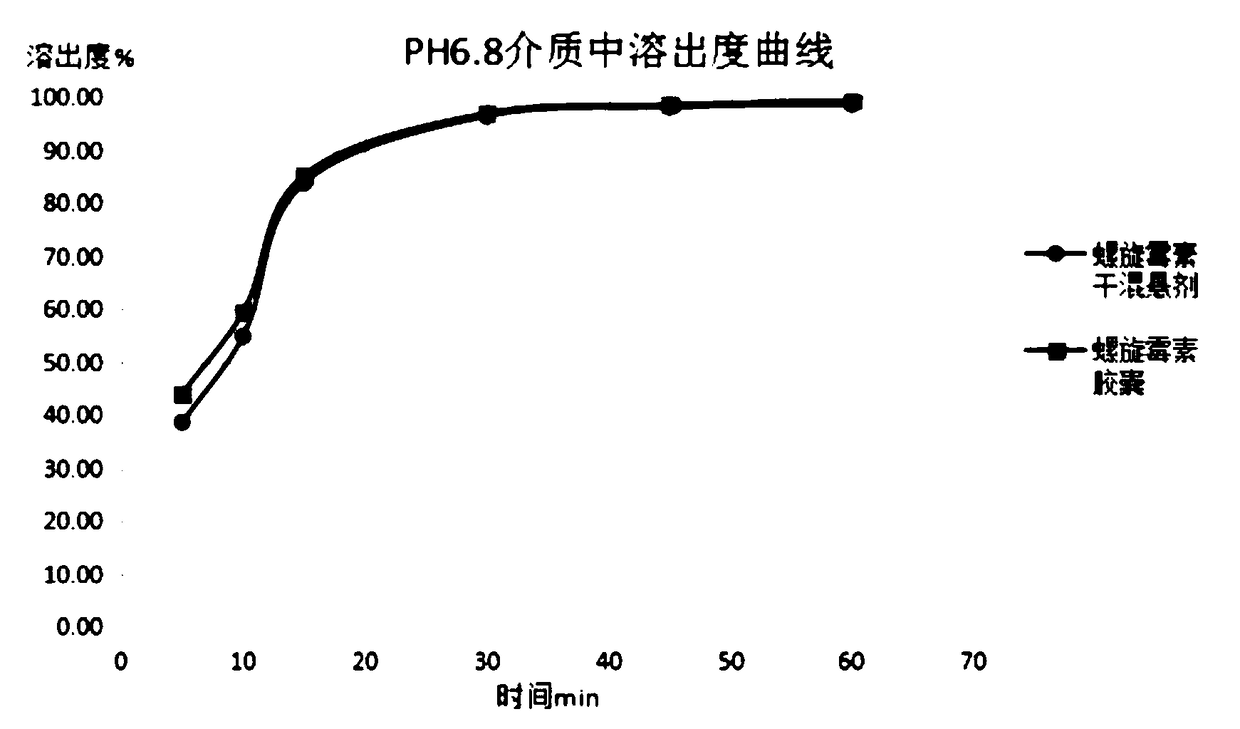
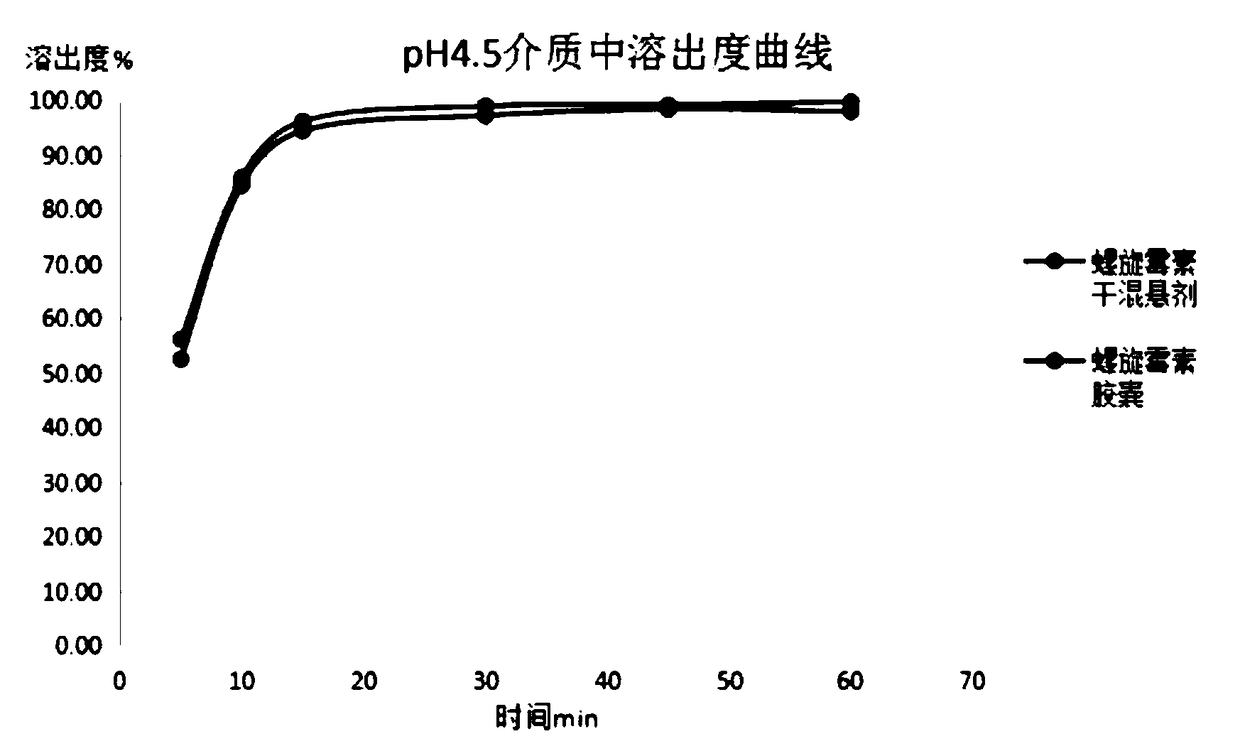
Spiramycin dry suspension and preparation method thereof
A technology of spiramycin and dry suspension, which is applied in the directions of antifungal agents, pharmaceutical formulations, medical preparations with inactive ingredients, etc., can solve the problems of small scope of applicable people, etc., and achieve the effect of solving bitterness and gritty feeling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] This embodiment discloses a kind of preparation method of spiramycin dry suspension:
[0030] A dry suspension of spiramycin consists of the following components:
[0031] 40-70 parts by weight of spiramycin, 10-25 parts by weight of lactose, 8-15 parts by weight of microcrystalline cellulose, 3-6 parts by weight of povidone, 2-5 parts by weight of colloidal silicon dioxide, magnesium stearate 2-5 parts by weight, 10-20 parts by weight of Eudragit, 2-5 parts by weight of stearic acid, 2-4 parts by weight of gum arabic, and 0.5-1 parts by weight of acesulfame potassium.
[0032] Prepared as follows:
[0033] (1) Place spiramycin, lactose, microcrystalline cellulose and colloidal silicon dioxide in a wet granulator and mix evenly, make a soft material with a 20% weight concentration of povidone aqueous solution, and place the soft material in an extrusion Extrude and spheronize in a spheronizer, dry the granules at 50°C, and take 60-80 mesh granules to obtain spiramycin...
Embodiment 2
[0037] This embodiment discloses the preparation method of another kind of spiramycin dry suspension:
[0038] A dry suspension of spiramycin consists of the following components:
[0039] 40-70 parts by weight of spiramycin, 10-25 parts by weight of lactose, 8-15 parts by weight of microcrystalline cellulose, 3-6 parts by weight of hydroxypropyl cellulose, 2-5 parts by weight of silicon dioxide, 2- 5 parts by weight, Eudragit 10-20 parts by weight, sodium lauryl sulfate 2-5 parts by weight, xanthan gum 2-4 parts by weight, sodium saccharin 0.5-1 parts by weight.
[0040] Prepared as follows:
[0041] (1) Put spiramycin, lactose, microcrystalline cellulose and silicon dioxide in a wet granulator and mix evenly, use 10% weight concentration of hydroxypropyl cellulose aqueous solution to make soft materials, and place the soft materials in extrusion Extrude and spheronize in a spheronizer, dry the granules at 60°C, and take 60-80 mesh granules to obtain spiramycin granules;
...
Embodiment 3
[0045] This embodiment discloses the preparation method of another kind of spiramycin dry suspension:
[0046] A dry suspension of spiramycin consists of the following components:
[0047] 40-70 parts by weight of spiramycin, 10-25 parts by weight of lactose, 8-15 parts by weight of microcrystalline cellulose, 3-6 parts by weight of ethyl cellulose suspension, 2-5 parts by weight of colloidal silicon dioxide, 2-5 parts by weight of talcum powder, 10-20 parts by weight of Eudragit, 2-5 parts by weight of sodium lauryl sulfate, 2-4 parts by weight of hydroxypropyl cellulose, and 0.5-1 part by weight of cyclamate.
[0048] Prepared as follows:
[0049] (1) Place spiramycin, lactose, microcrystalline cellulose and colloidal silicon dioxide in a wet granulator and mix them evenly, and use 15% weight concentration of ethyl cellulose suspension aqueous solution to make soft material, soft material Put it in an extrusion spheronizer, extrude and spheronize, dry the granules at 55°C,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com