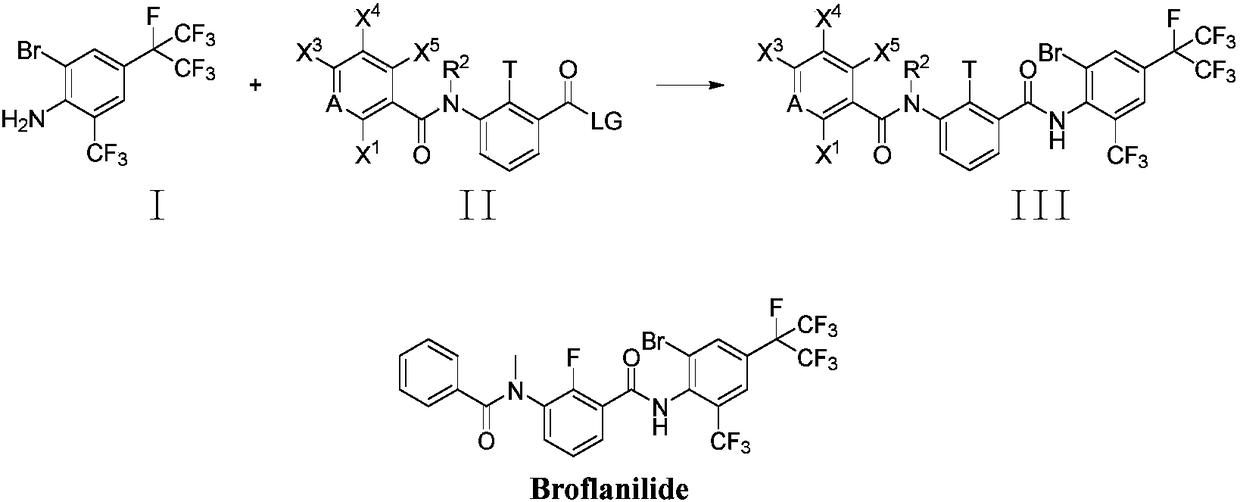
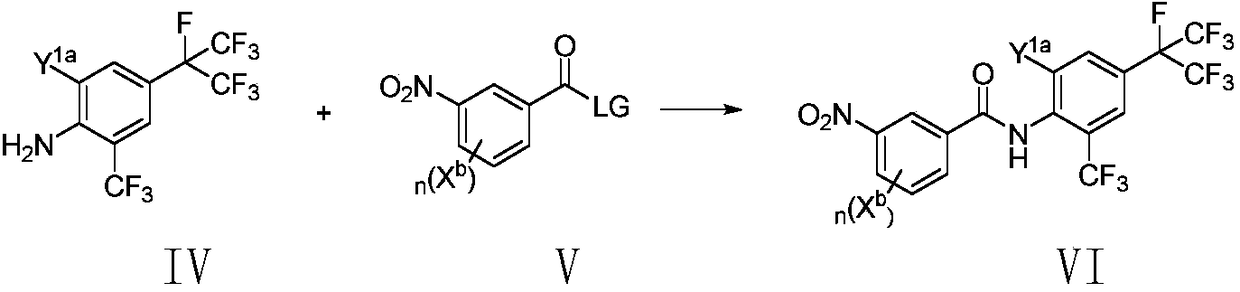
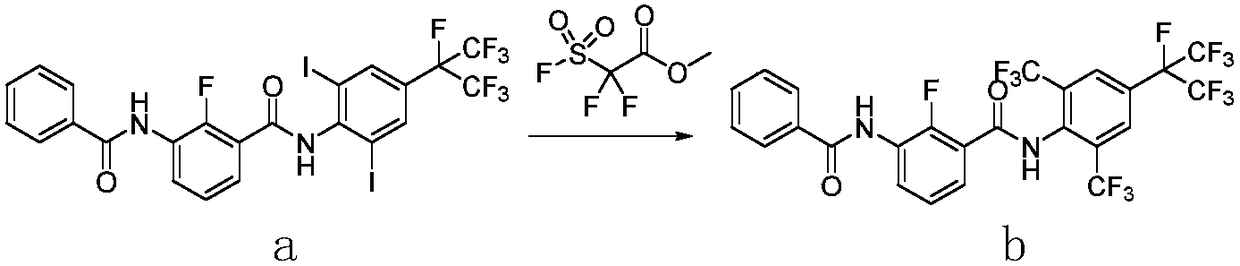
Method for preparing o-trifluoromethylaniline compound and intermediate thereof
A technology of trifluoromethylaniline and trifluoromethylation, applied in the field of preparation of o-trifluoromethylaniline compounds, achieving high yield, mild reaction conditions and good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] 1) Preparation of N-(2-iodo-6-bromo-4-heptafluoroisopropylphenyl)-2-fluoro-3-(N-methylbenzamido)benzamide (IX):
[0066]
[0067] 2-iodo-6-bromo-4-heptafluoroisopropylaniline (4.70g, 10.0mmol) was dissolved in acetonitrile (40mL), potassium iodide (0.42g, 2.5mmol) was added, and 2 -Fluoro-3-(N-methylbenzamido)benzoyl chloride (5.90 g, 20.0 mmol), heated to 85° C. for reflux reaction, monitored by TLC until the reaction was complete. The reaction solution was cooled to room temperature, filtered to remove insoluble matter, the filtrate was distilled under reduced pressure, the residue was dissolved in ethyl acetate (50 mL), washed three times with 10% sodium hydroxide solution, the aqueous phase was recovered, and the organic phase was sequentially washed with 1 mol / L Wash with hydrochloric acid and saturated brine, dry over anhydrous magnesium sulfate, filter, distill off ethyl acetate under reduced pressure, the residue is separated and purified by column chromatogr...
Embodiment 2
[0084] 1) Preparation of N-(2-iodo-6-bromo-4-heptafluoroisopropylphenyl)-2-fluoro-3-nitrobenzamide (IX):
[0085]
[0086] Dissolve 2-iodo-6-bromo-4-heptafluoroisopropylaniline (4.70g, 10.0mmol) in 40mL of acetonitrile, add potassium iodide (0.42g, 2.5mmol), and then add 2-fluoroisopropylaniline to the reaction solution -3-Nitrobenzoyl chloride (4.12g, 20.0mmol), the mixture was heated to 85°C for reflux reaction, and TLC monitored until the reaction was complete. The reaction solution was cooled to room temperature, filtered to remove insoluble matter, acetonitrile was distilled off under reduced pressure, and 50 mL of ethyl acetate was added to the residue. First, the organic phase was washed three times with 10% sodium hydroxide solution, and the aqueous phase was reclaimed, then the organic phase was washed with 1mol / L hydrochloric acid and saturated brine successively, dried over anhydrous magnesium sulfate, filtered, and ethyl acetate was distilled off under reduced p...
Embodiment 3
[0101] 1) Preparation of N-(2-iodo-6-bromo-4-heptafluoroisopropylphenyl)-2-fluoro-3-benzamidobenzamide (IX):
[0102]
[0103] Dissolve 2-iodo-6-bromo-4-heptafluoroisopropylaniline (4.70g, 10.0mmol) in N,N-dimethylformamide (40mL), add sodium iodide (0.38g, 2.5mmol ), and then added 2-fluoro-3-benzamidobenzoyl chloride (5.61g, 20.0mmol) to the reaction solution, heated to 85°C for reflux reaction, and monitored by TLC until the reaction was complete. The reaction solution was cooled to room temperature, filtered to remove insoluble matter, the filtrate was distilled under reduced pressure, the residue was dissolved in ethyl acetate (50 mL), washed three times with 10% sodium hydroxide solution, the aqueous phase was recovered, and the organic phase was sequentially washed with 1 mol / L Wash with hydrochloric acid and saturated brine, dry over anhydrous magnesium sulfate, filter, evaporate ethyl acetate under reduced pressure, and separate and purify the residue by column chr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com