A method of converting human hematopoietic progenitor cells into hematopoietic stem cells
A technology of hematopoietic progenitor cells and hematopoietic stem cells, applied in the field of hematopoietic stem cells, can solve the problems of low number of hematopoietic stem cells, increase the fatality rate of opportunistic infections, etc., and achieve the effect of high number
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1: Using the special transformation medium for human umbilical cord blood hematopoietic stem cells to obtain a large number of umbilical cord blood hematopoietic stem cells from hematopoietic progenitor cells, including the following steps:
[0024] 1. Obtain peripheral blood mononuclear cells (PBMC).
[0025] (1) Collect 80-120ml of umbilical cord blood with a disposable blood bag (containing anticoagulants such as heparin sodium), transfer the umbilical cord blood from the blood bag to a 500ml culture bottle, add normal saline to dilute 2-3 times, mix well and gradually Add dropwise to 0.4 times the volume of lymphocyte separation medium, be careful not to damage the interface.
[0026] (2) Centrifuge at 1500-2000rpm / min for 20 minutes. Due to different densities, the centrifuge tube is divided into four layers from top to bottom: the first layer is the plasma layer, the second layer is the ring-shaped milky white mononuclear cell layer (PBMC), the The thi...
Embodiment 2
[0041] Embodiment 2: The above-mentioned isolated human placental blood hematopoietic stem cells are subjected to phenotype identification, activity rate and purity detection and functional identification, including the following steps:
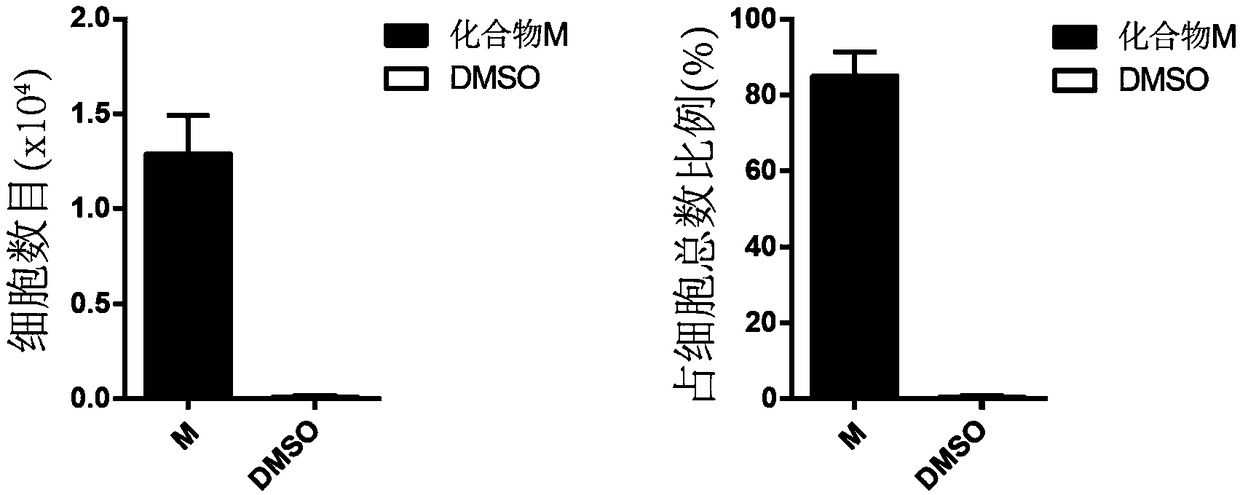
[0042] 1. Cell count
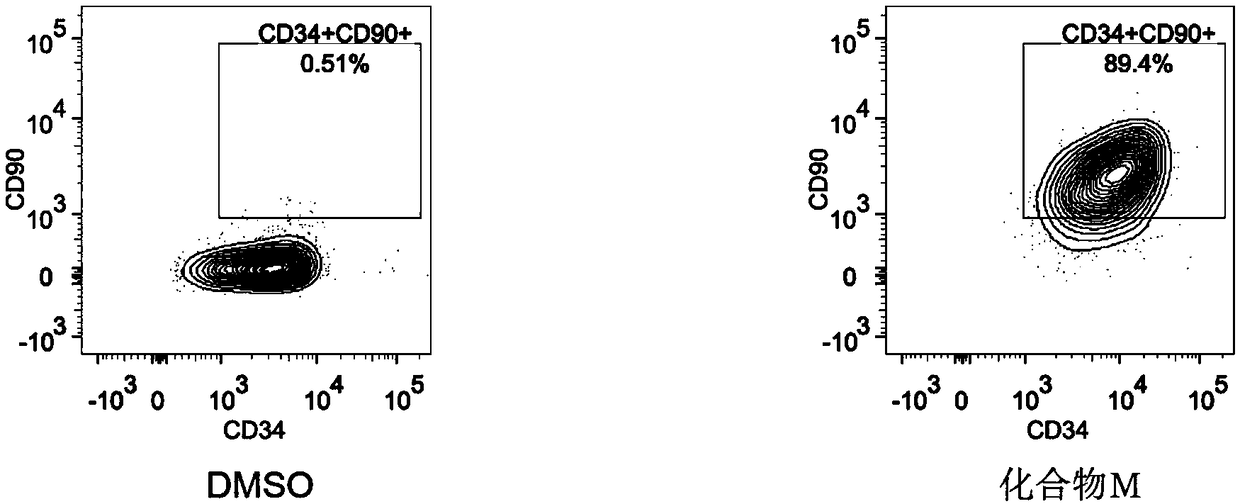
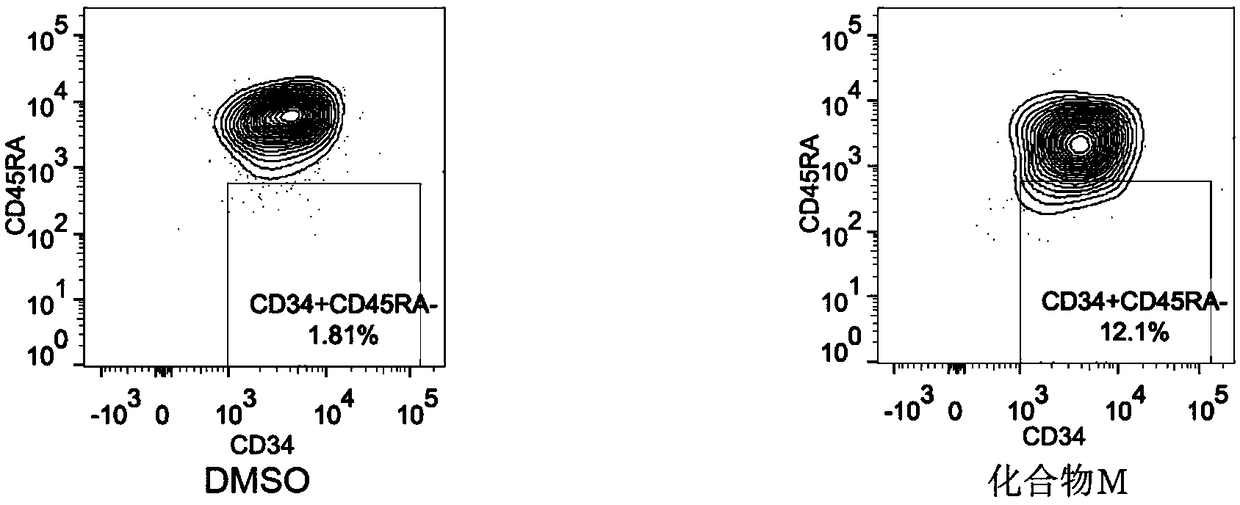
[0043] Count the number of CD34+CD90+ and CD34+CD45RA- hematopoietic stem cells after culture respectively.
[0044] Table 2 Statistics of cell number after culture of CD34+CD90- hematopoietic progenitor cells
[0045]
[0046] Table 3 Statistics of cell number after culture of CD34+CD45RA+ hematopoietic progenitor cells
[0047]
[0048] 2. Cell Flow Cytometry Analysis
[0049] Using BD’s FACS Verse flow detector, take 20 μl of cell suspension and add 0.2 μl of FITC-labeled CD34, PE-labeled CD38, APC-Cy7-labeled CD45RA, and APC-labeled CD90 dissolved in 0.5% BSA. After vortexing each tube, incubate at room temperature in the dark for 15 minutes, add an appropriate amount of PBS, centrifuge at 1600 rpm for 5 mi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com