Detection kit for cardiac troponin I and preparation method thereof
A technique for cardiac troponin and detection kit, which is applied in biological tests, measuring devices, material inspection products, etc., can solve the problems of low sensitivity, long detection period, and small detection concentration range, so as to improve sensitivity and ensure accuracy. The effect of performance and precision, efficient detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] The preparation of embodiment 1 detection kit
[0061] 1. Preparation of the sample pad: use glass fiber membrane as the sample pad, place the glass cellulose membrane on a white PVC board, prepare 0.1% Tween-20 solution as the sample pad treatment solution, and use 45 μL / cm 2 Sprinkle the sample pad treatment solution evenly on the glass cellulose membrane, spread evenly on the front and back of the nitrocellulose membrane until there are no white spots, and then place it on the screen window to balance at room temperature for 60min±15min. , dried at 37°C for 30-72 hours;
[0062] 2. Preparation of reaction solution:
[0063] (1) Polypropylene fluorescent microspheres (using Eu as the fluorescent substance, the particle size of the fluorescent microspheres is 200nm, and the fluorescent microspheres contain carboxyl modification) diluted 10 times with 20mM MES (2-morpholineethanesulfonic acid) (pH 6.0) , ultrasonic 1min;
[0064] (2) Prepare 5mg / mL EDC (1-(3-dimethyl...
Embodiment 2
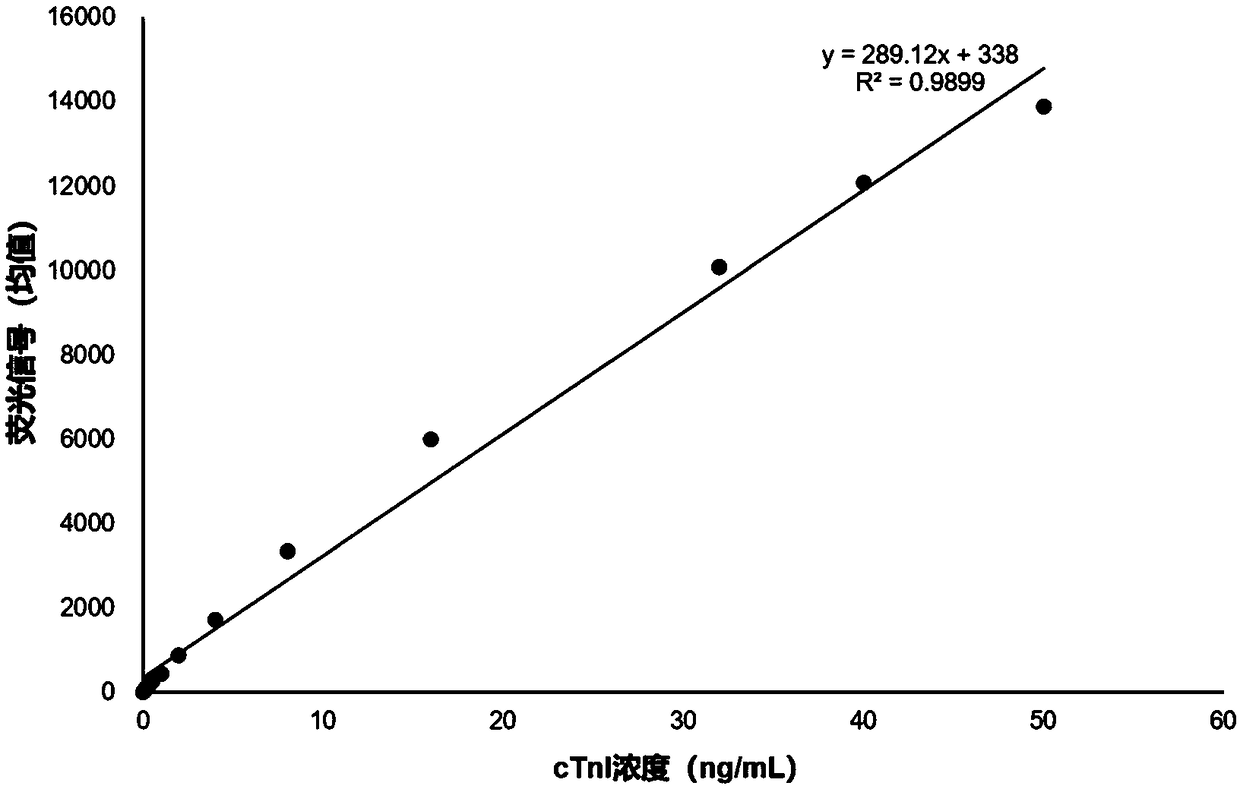
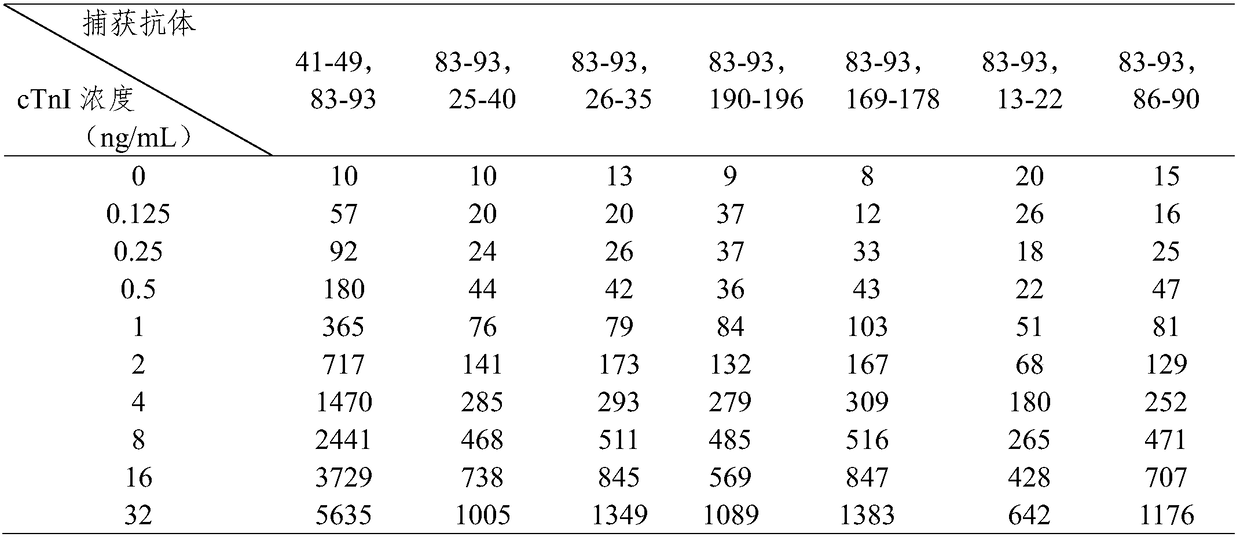
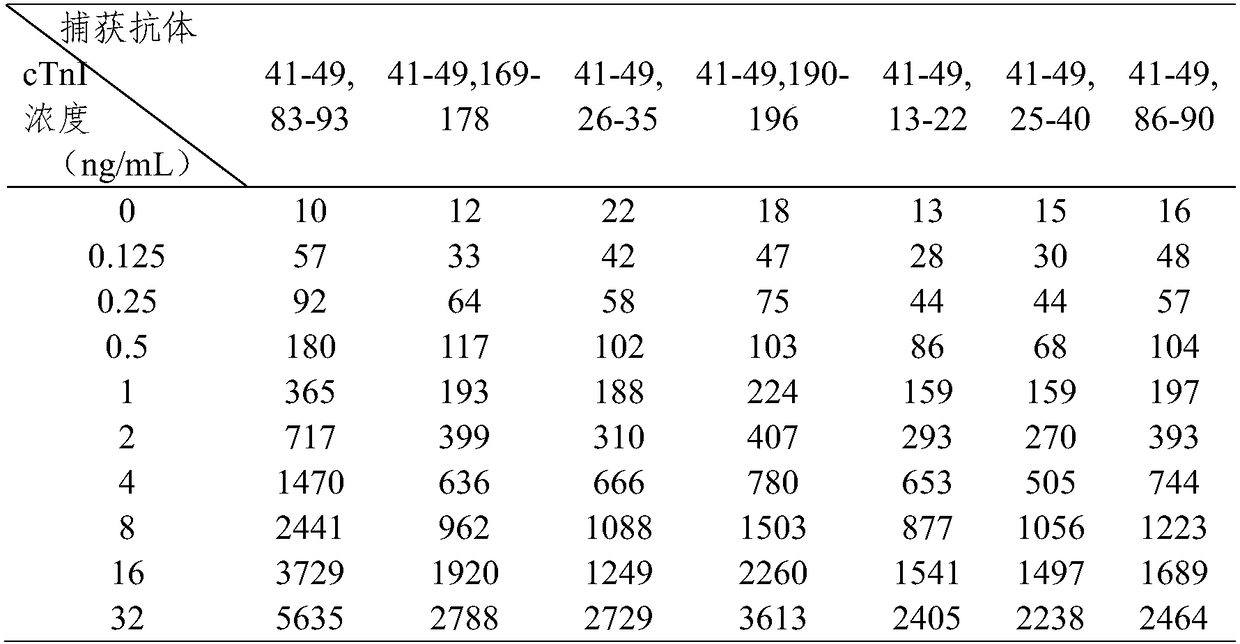
[0079] The sensitivity analysis that embodiment 2 kit detects
[0080] 1. Preparation of cTnI detection standard
[0081] Use normal human serum as a diluent to prepare cTnI antigen standard products with concentrations of 0 ng / mL and 50 ng / mL respectively; mix the two concentrations of cTnI standard product solutions in sequence according to a certain concentration, and prepare 0-50 ng / mL respectively Concentration gradient of cTnI detection standard.
[0082] 2. Detection reaction
[0083] Dilute the reaction solution in the kit prepared in Example 1 by 10 times with the diluent, add 60 μL of the detection standard of each concentration obtained in step 1, and mix well for 5 seconds to 10 seconds.
[0084] Pipette 90 μL of the mixture of the detection standard and the reaction solution at each concentration above, and add it dropwise to the sample hole on the sample pad of the test paper card. Be careful not to inhale air bubbles when sampling, and place it at room tempera...
Embodiment 3
[0090] Accuracy and precision analysis of embodiment 3 kit detection
[0091] The kit prepared in Example 1 was used to detect cTnI in 3 clinical serum samples. According to the content of cTnI in the three samples, they are divided into three groups: low value, middle value and high value, among which the clinical measurement value of the low value fixed value sample is 0.15ng / mL, and the clinical measurement value of the middle value fixed value sample is 0.57ng / mL , the clinical measurement value of high-value samples was l.89ng / mL. Each of the three fixed-value samples was tested 10 times, and the test results are shown in Table 2. The results show that the test kit prepared in Example 1 is used to detect 3 samples of fixed value, and the test results are very close to the clinical measurement values (the difference between the average value of 10 tests and the clinical measurement values is only about 0.01 ng / mL), It shows that the cTnI detection kit of the present ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Width | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com