A phosphorescent polymer probe capable of detecting peroxynitrite by ratiometric method and its preparation and application
A technology of peroxynitrite and polymer, which is applied in the direction of fluorescence/phosphorescence, material analysis by optical means, measuring devices, etc. It can solve the problems of difficult mediation process and short life of peroxynitrite, and achieve good results. Water solubility and biocompatibility, low biological toxicity, and the effect of improving the detection signal-to-noise ratio
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: the preparation of the phosphorescence iridium complex Ir2 that can detect peroxynitrite
[0042]
[0043] The specific operation steps are:
[0044] Preparation of Compound 2: Add 1 (3.0 mmol) into a round-bottomed flask, vacuumize and change nitrogen three times to remove water in the air; subsequently, dry dichloromethane, triethylamine (0.5 mL) and Tert-butyldiphenylchlorosilane (4.5mmol), stirred at room temperature for 6 hours; after the reaction, filter off the white precipitate, take the filtrate and spin off the organic solvent, column chromatography to obtain light yellow oily liquid 2, yield 80% .
[0045] 1 H NMR (400MHz, CDCl 3 ):7.78(dd, J=8.0Hz, 2.4Hz, 4H), 7.72(d, J=2.8Hz, 1H), 7.66(dd, J=8.8Hz, 2.8Hz, 1H), 7.47-7.40(m, 6H),6.80(d,J=8.8Hz,1H),3.68(s,3H),1.22(s,9H).δ(ppm): 13 C NMR (100MHz, CDCl 3 )δ (ppm): 151.3, 150.6, 142.1, 135.3, 132.4, 130.2, 127.9, 119.5, 117.3, 107.3, 55.6, 26.6, 19.9.
[0046]Preparation of compound 3: Add...
Embodiment 2
[0054] Example 2: Preparation of a complex water-soluble polymer probe P-ONOO sensitive to peroxynitrite
[0055]
[0056] Among them, a, b, c are natural numbers;
[0057] The specific operation steps are: add Ir1 (0.03mmol), Ir2 (0.3mmol), AIBN (0.3mmol), pyrrolidone (10.0mmol) and a small amount of tetrahydrofuran (5mL) to the schlenk reaction tube; freezing-pumping-thawing, and so on for three times , heated to 70° C. and stirred and refluxed for 6 hours; after the reaction stopped, it was settled three times with ether, and P was obtained by suction filtration with a yield of 32%.
Embodiment 3
[0058] Embodiment 3: complexes Ir1, Ir2 are tested to peroxynitrite responsive emission spectrum
[0059] The spectrum test concentration of the iridium complexes Ir1 and Ir2 used in the present invention is 10 μM, and the test solvent is PBS solution mixed with 1% DMSO. When the emission spectrum is measured, the excitation wavelength is 605 nm.
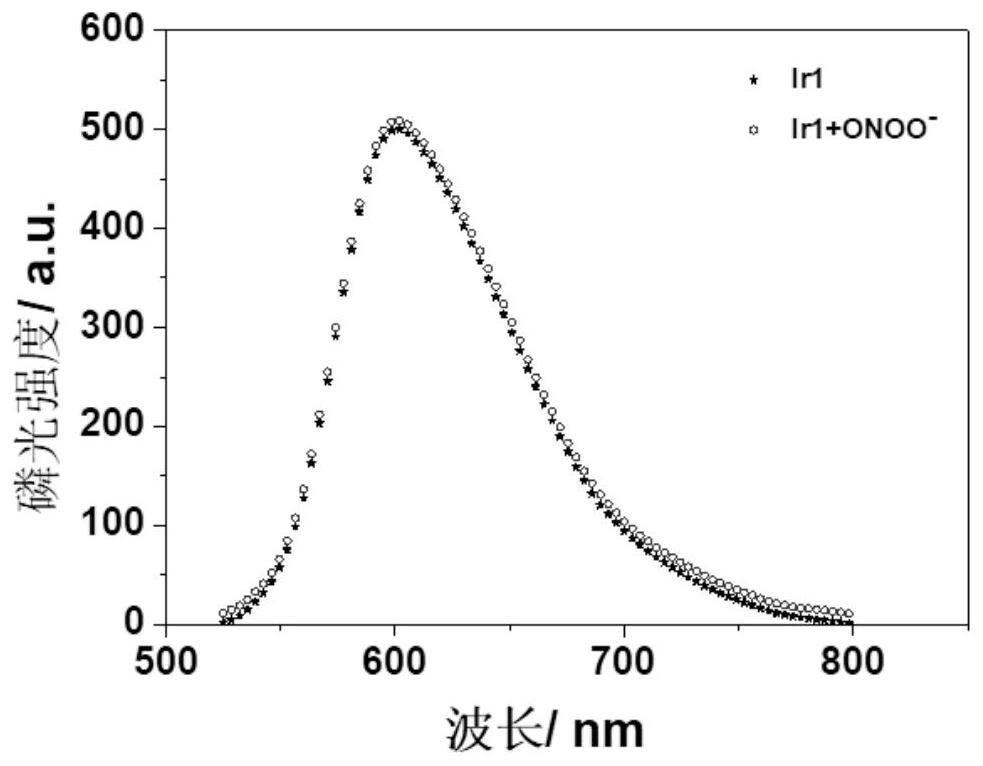
[0060] The emission spectrum results of the iridium complex Ir1 with and without peroxynitrite conditions are as follows: figure 1 As shown, it can be seen from the figure that the emission wavelength of the complex Ir1 is 605nm in the presence or absence of peroxynitrite, and the luminous intensity is almost unchanged with the change of the concentration of peroxynitrite. It shows that the iridium complex Ir1 can be used as a reference in the detection of peroxynitrite, and the ratio method can be constructed with Ir2, which can reduce unnecessary interference in the environment and increase the accuracy of detection.
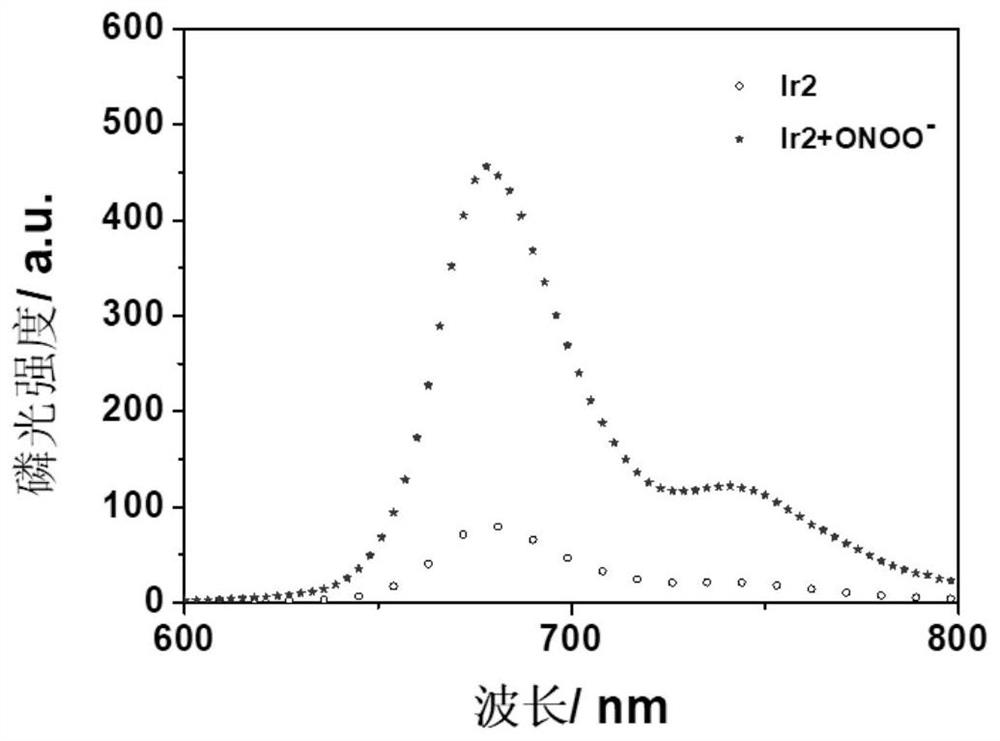
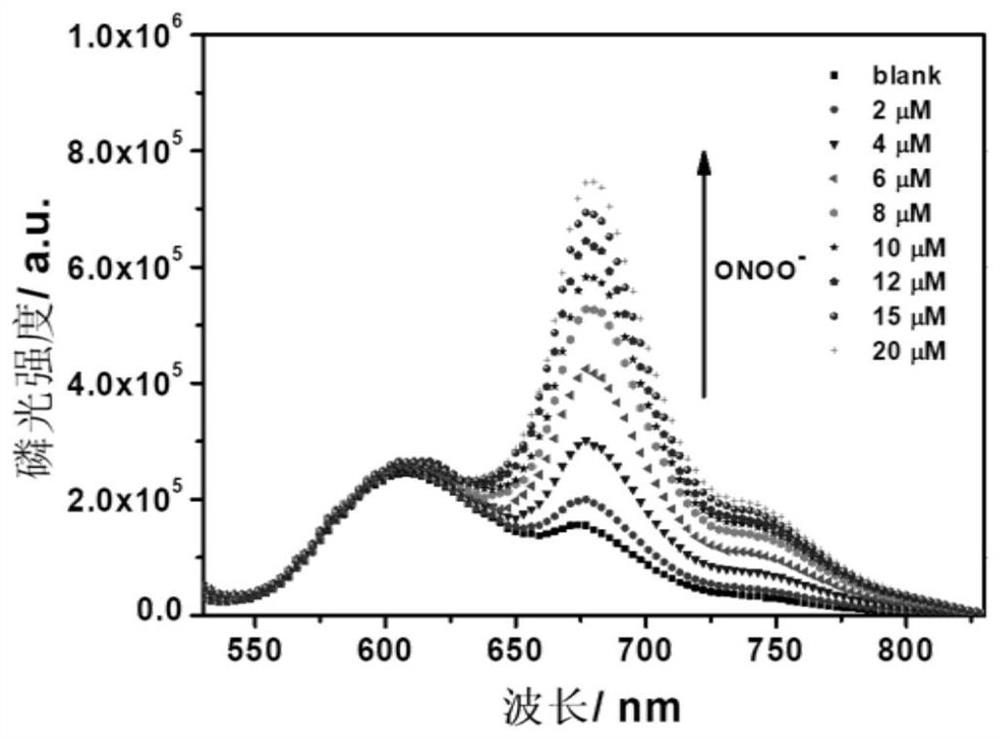
[0061] The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com