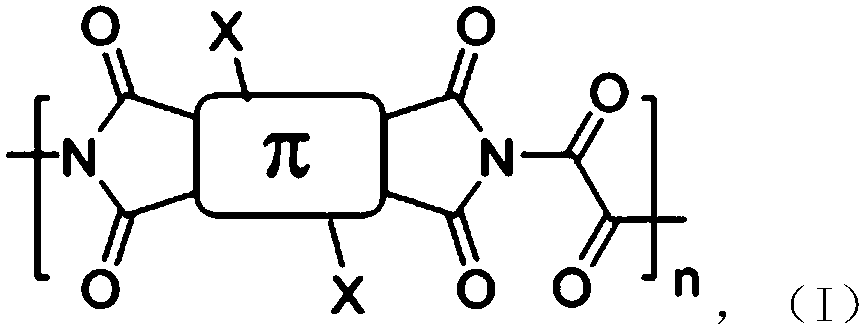
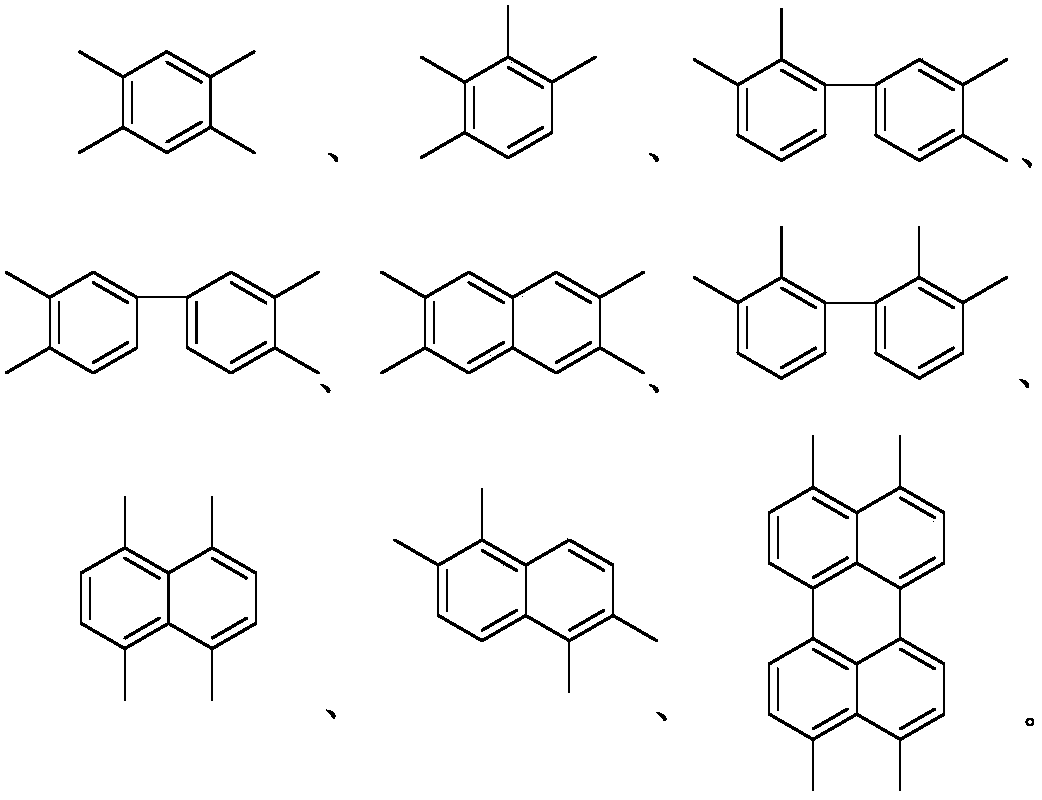
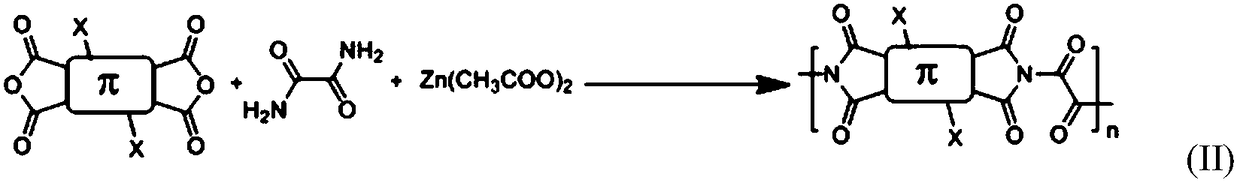A class of rapid charge and discharge positive electrode active materials, preparation method and applications thereof
A cathode active material, charging and discharging technology, applied in battery electrodes, circuits, electrical components, etc., can solve the problems of low specific capacity of electrode materials, low utilization rate of active sites, increase energy density, etc., and achieve excellent cycle stability performance , excellent electrochemical performance, the effect of improving rate performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] A fast charge-discharge positive electrode active material, and its preparation method includes the following steps:
[0034] Pyromellitic dianhydride (873mg, 4mmol), oxamide (360.33mg, 4mmol), zinc acetate (807.257mg, 4.4mmol) and imidazole (10g) were added to a 100ml single-necked flask in sequence, stirred and heated to 90°C. After the imidazole is fully melted and continue to heat up to 150°C, keep stirring and react for 24h; after the reaction is over, stop heating, and the system is naturally cooled to room temperature, then wash with water, dry the filter cake, wash the filter cake with dimethyl sulfoxide, and vacuum dry, The product was 1.04 g, with a yield of 86%.
Embodiment 2
[0036] A fast charge-discharge positive electrode active material, and its preparation method includes the following steps:
[0037] Add naphthalene tetracarboxylic dianhydride (1.07g, 4mmol), oxamide (324.297mg, 3.6mmol), zinc acetate (733.87mg, 4mmol) and imidazole (10g) into a 100ml single-necked flask in sequence, stir and warm to 90°C. After the imidazole is fully melted, the temperature is continued to be increased to 150° C., and the reaction is kept under stirring for 24 hours; after the reaction is completed, the heating is stopped, the system is naturally cooled to room temperature, and then washed with water, and the filter cake is dried. The filter cake was washed with dimethyl sulfoxide and dried in vacuum. The final product was 1.12 g with a yield of 79%.
Embodiment 3
[0039] A fast charge-discharge positive electrode active material, and its preparation method includes the following steps:
[0040] Add perylene tetracarboxylic dianhydride (1.57g, 4mmol), oxamide (360.33mg, 4mmol), zinc acetate (733.87mg, 4mmol) and imidazole (10g) into a 100ml single-necked flask, stir and warm up to 90℃, and wait for the imidazole After being fully melted, continue to heat up to 150°C and keep stirring for 24 hours. After the reaction is over, stop heating. After the system is naturally cooled to room temperature, wash with water, dry the filter cake, wash the filter cake with dimethyl sulfoxide, and vacuum dry. The final product 1.37g, the yield is 72%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com