Method for establishing HT-29 cell inflammatory model and application
A technology of HT-29, an inflammation model, applied in the biological field, can solve the problem that the mechanism of action needs to be studied, and achieve a good therapeutic effect and a long time-consuming effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] A kind of establishment method of HT-29 cell inflammation model
[0024] 1. Cell culture
[0025] HT-29 cell culture medium: DMEM high glucose medium added with 10% FBS; culture conditions: carbon dioxide concentration 5%, temperature 37°C.
[0026] 2. Cell Processing
[0027] Inoculate HT-29 cells in a 24-well plate and culture overnight. After the cells grow on the wall, replace the medium and add human TNF-α (20mg / ml). After co-cultivating for 12 hours, add LPS to the medium (1 μg / ml), cultivated for 15 hours.
[0028] 3. Result detection
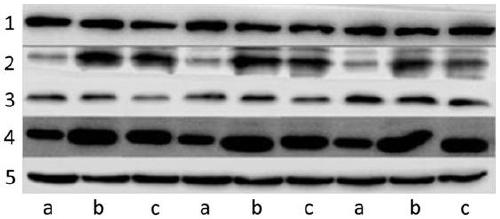
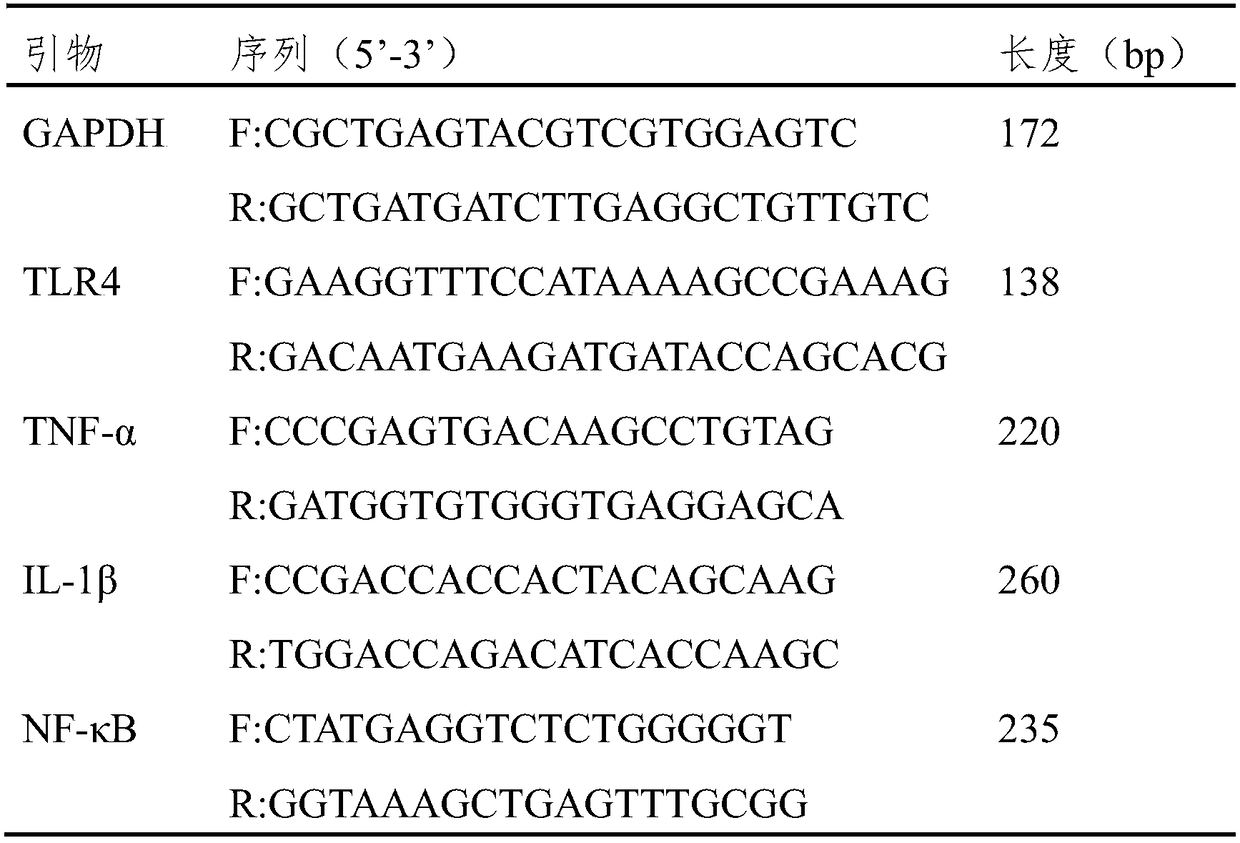
[0029] 3.1 Real-time fluorescent quantitative PCR (real-time PCR) detection of TLR4, NF-κB, TNF-α and IL-1β mRNA expression in HT-29 cells
[0030] 3.1.1 Extraction of total cellular RNA
[0031] (1) After digesting the cells with trypsin, transfer the cell suspension together to a nuclease-free centrifuge tube, centrifuge at 500g, 4°C for 5 minutes, discard the supernatant, and collect the precipitated cells;
[0032] (2) A...
Embodiment 2
[0094] Effect of probiotic agent VSL#3 on HT-29 cell inflammation model
[0095] 1. Implementation steps
[0096] The cells were plated in T-25 flasks, and after the cells adhered to the wall, they were processed according to the following groups:
[0097] Group A: Blank control group: 90% DMEM high glucose medium + 10% FBS cultured for 24 hours;
[0098] Group B: model control group: after giving Human TNF-α (20mg / ml) for incubation for 12 hours, then giving LPS (1ug / ml) for incubation for 15 hours;
[0099] Group C: VSL#3 treatment group: after the same treatment as the model group, probiotic agent VSL#3 (1×10 5 cfu / ml) were co-incubated for 7 days.
[0100] 2. Test results
[0101] According to the detection steps of Example 1, the mRNA and protein of HT-29 cells were extracted respectively, and the expression levels of TLR4, NF-κB, TNF-α and IL-1β in cells were detected and compared. The result looks like this:
[0102] Table 3 mRNA relative differential expression f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com