Reagent, device and method for drug-resistant heterogeneous circulating tumor cell capture and gene analysis
A tumor cell and capture reagent technology, applied in the field of drug-resistant heterogeneous circulating tumor cell capture and gene analysis, can solve the problems of unrealized heterogeneous circulating tumor cell capture, complex blood components, etc., to improve sensitivity and Accuracy, effective capture of enrichment, the effect of increasing concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
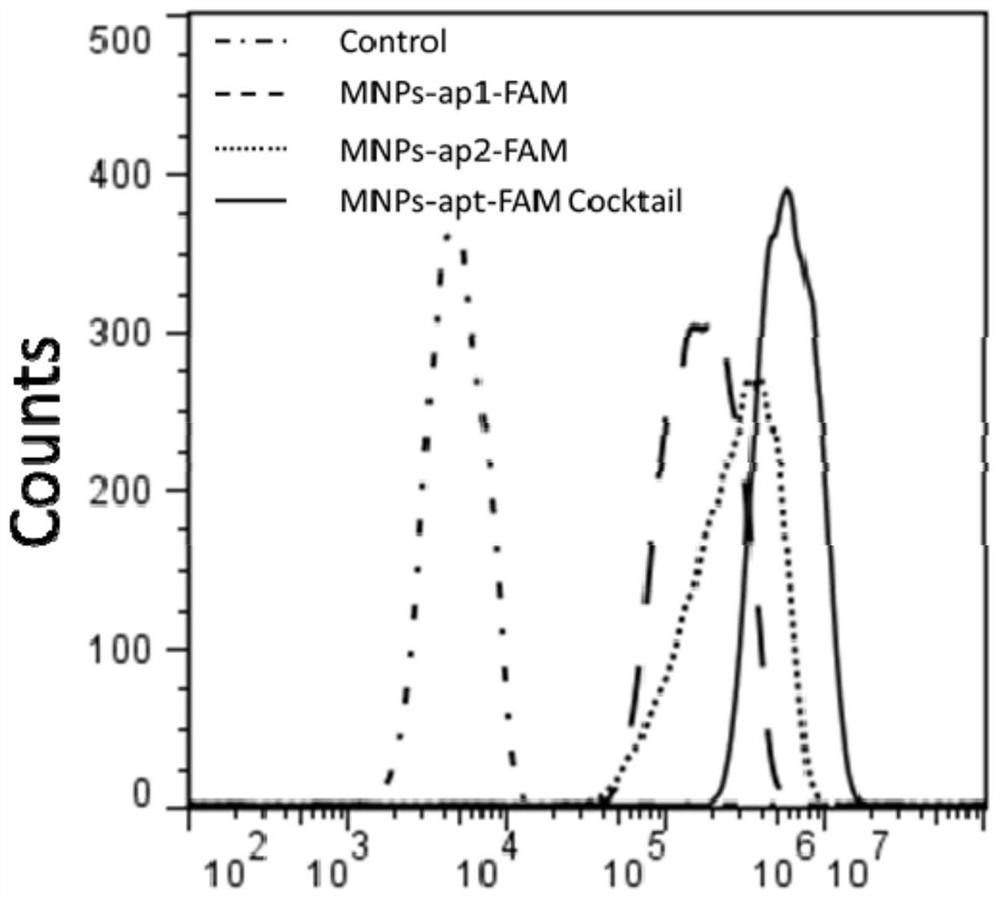
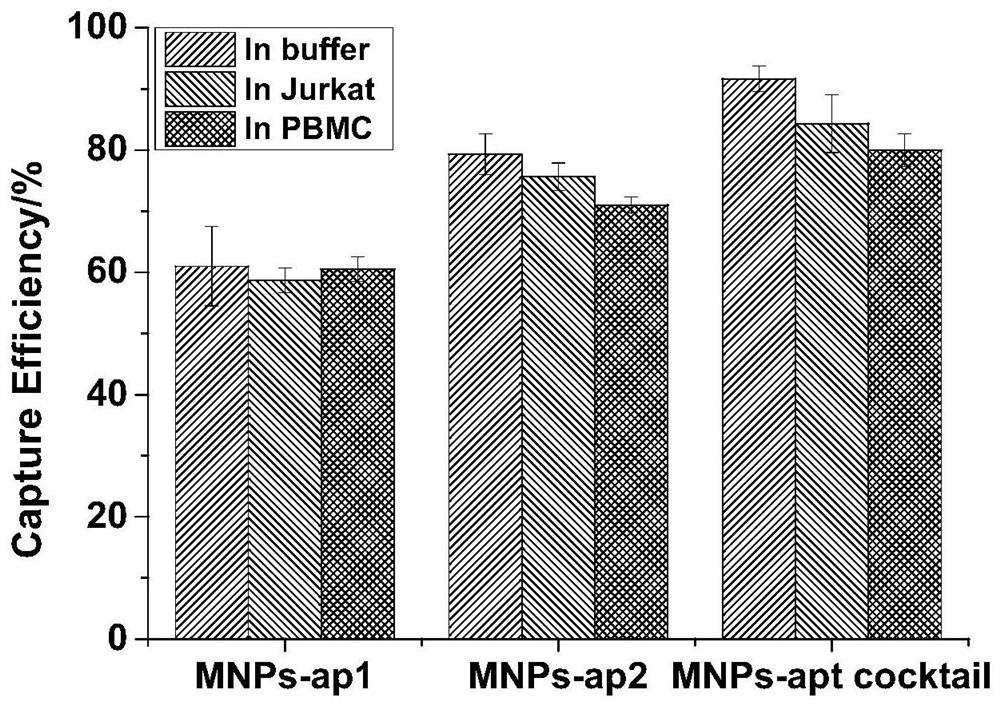
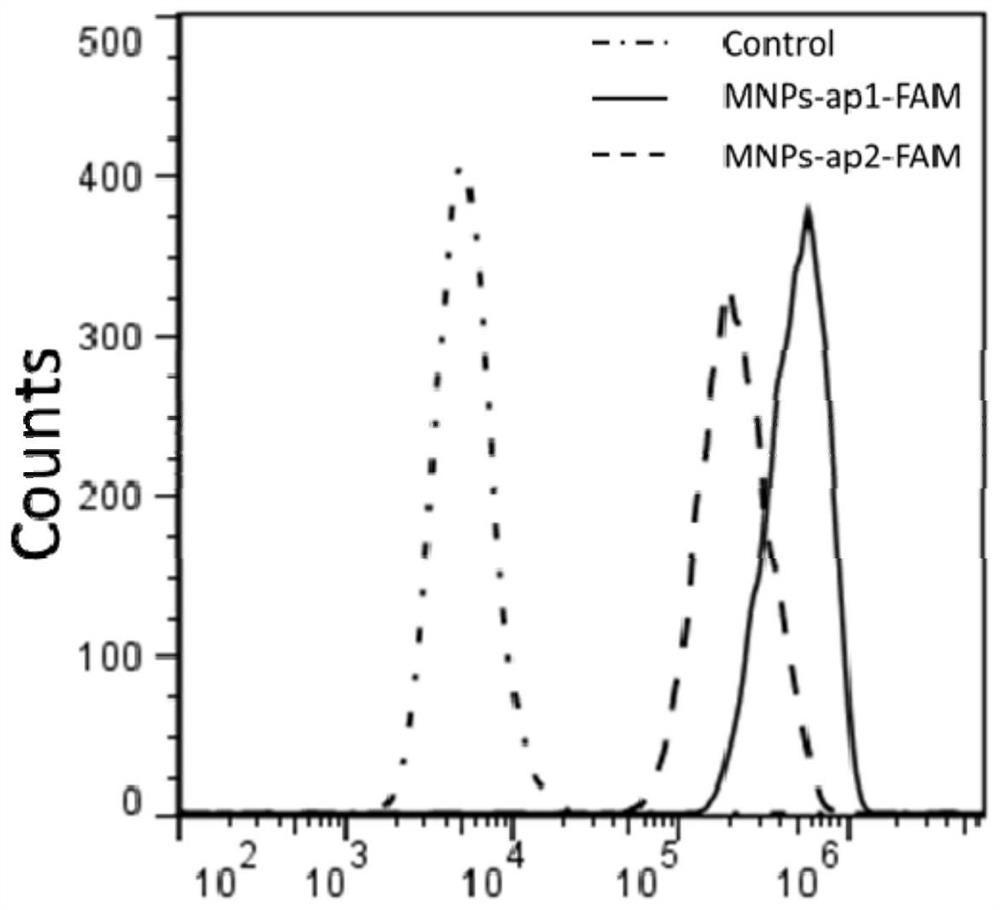
Image
Examples
Embodiment 1
[0060] Example 1: Nucleic acid aptamer-modified magnetic bead capture and microwell chip purification of circulating tumor cells
[0061] Step 1: preparing aptamer-modified magnetic beads;
[0062] Step 1.1: Two kinds of biotin-modified nucleic acid aptamers were used to incubate with streptavidin-modified magnetic beads respectively, and the incubation conditions were 25°C, 150rpm, 30 minutes;
[0063] The nucleic acid aptamer ap1 sequence is as follows:
[0064] 5'-TTTATGGGTGGGTGGGGGGTTTTT-3' (SEQ ID NO: 1)
[0065] The nucleic acid aptamer ap2 sequence is as follows:
[0066] 5'-GGTTGGTTGGGGTTGGGTTGTTTTTGGGGTGATATGGGGGTTGGA-3'
[0067] (SEQ ID NO:2)
[0068] Step 1.2: Add 10uL of 10% bovine serum albumin to step 1.1, 25°C, 150rpm, 30 minutes;
[0069] Step 1.3: Separate and wash the mixed solution in step 1.2 under an external magnetic field.
[0070] After the above three steps, the aptamer-modified magnetic beads are obtained.
[0071] Step 2: capture of circulatin...
Embodiment 2
[0088] Example 2: Genetic Analysis of Circulating Tumor Cells
[0089] Step 1: For the circulating tumor cells captured and purified in Example 1, a single circulating tumor cell (Hoechst 33258+ / CK+ / CD45-) was extracted by micromanipulation under a fluorescent microscope;
[0090] Step 2: Use the single cell whole gene amplification kit (Sigma) to perform whole gene amplification on the extracted circulating tumor cells, and refer to the kit instruction manual for detailed steps;
[0091] Step 3: Perform electrophoresis analysis on the sequence amplified in step 2, the results are as follows Figure 7 a;
[0092] Step 4: Use the sequence amplified in step 2 as a template to amplify the specific site, and the electrophoresis analysis results are as follows: Figure 7 b and Figure 7 c;
[0093] Primer sequence (5'-3'):
[0094] KRAS-Forward AAGGTACTGGTGGAGTATTTG (SEQ ID NO: 3)
[0095] KRAS-Reverse GTACTCATGAAAATGGTCAGAG (SEQ ID NO: 4)
[0096] EGFR19-Forward GTGCATCGCTGGT...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com