Ornidazole orally disintegrating tablet and preparation method thereof
A technology of ornidazole mouth and ornidazole, which is applied in the field of pharmaceutical preparations, can solve the problems of strong irritation, great harm to human body, and adverse reactions, and achieve the effect of rapid disintegration, reliable curative effect, and no gritty feeling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
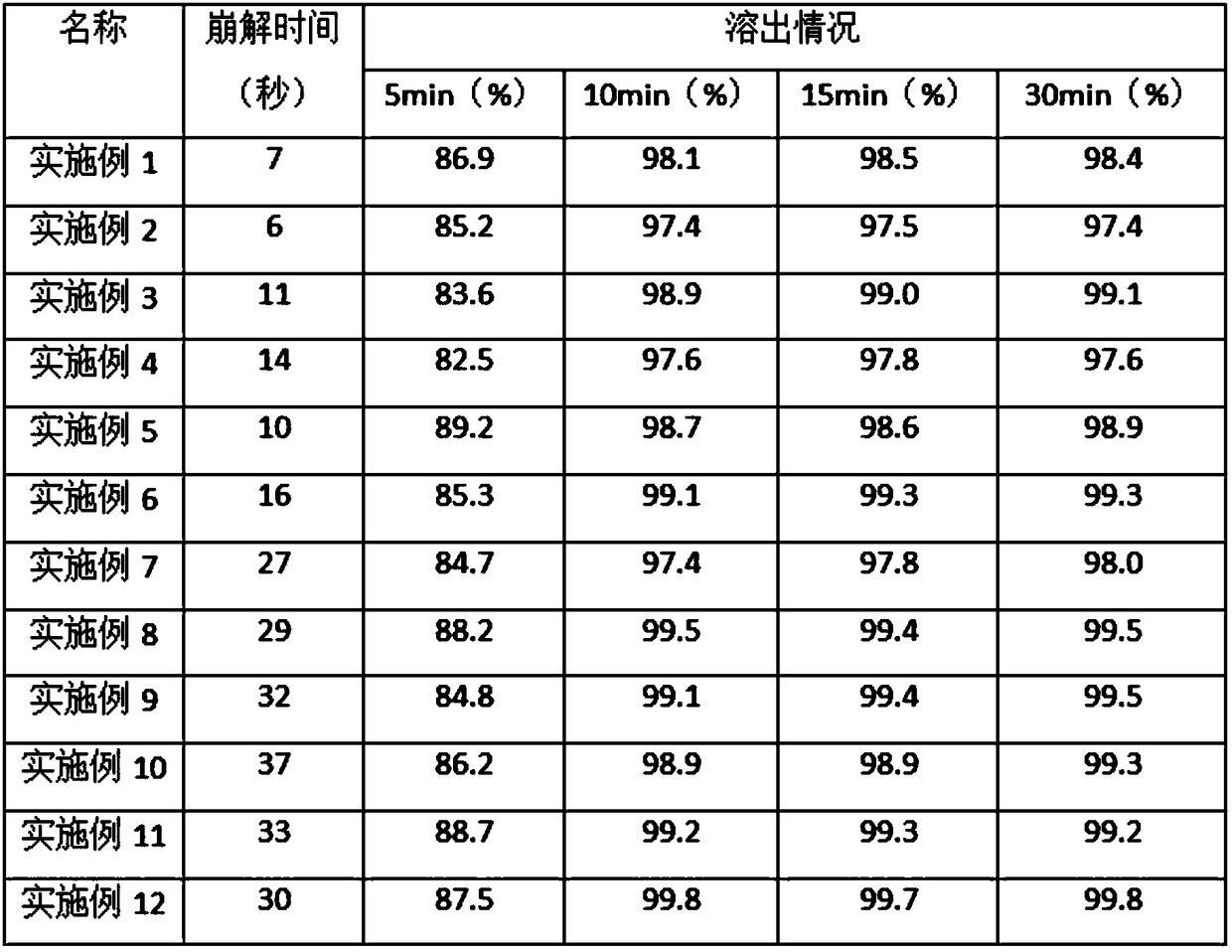
Embodiment 1
[0049] A kind of ornidazole orally disintegrating tablet adopts wet granulation and is made of components comprising the following weight percentages:
[0050] Ornidazole 250g (50%),
[0051] Crospovidone 150g (30%),
[0052] Sodium carboxymethylcellulose 5g (1%),
[0053] Sodium saccharin 40g (8%),
[0054] Mannitol 45g (9%),
[0055] Calcium stearate 10g (2%);
[0056] The preparation method of ornidazole orally disintegrating tablet in the present embodiment, comprises the steps:
[0057] (1) Pass ornidazole through a 60-mesh sieve, and grind the rest of the auxiliary materials through a 100-mesh sieve.
[0058] (2) after ornidazole, sodium saccharin, mannitol, crospovidone of 15% in the proportioning of raw materials are sieved and mixed uniformly, add the concentration of sodium carboxymethylcellulose that is 25% in the proportioning of raw materials Aqueous solution to prepare soft materials;
[0059] (3) pass through a 20-mesh sieve to granulate, dry in an oven a...
Embodiment 2
[0063] An ornidazole orally disintegrating tablet, which is dry granulated, is made of components comprising the following weight percentages:
[0064] Ornidazole 250g (50%),
[0065] Low-substituted hydroxypropyl cellulose 140g (28%),
[0066] Sodium saccharin 55g (11%),
[0067] Xylitol 30g (6%),
[0068] Calcium stearate 15g (3%),
[0069] Stearic acid 10g (2%);
[0070] The preparation method of ornidazole orally disintegrating tablet in the present embodiment, comprises the steps:
[0071] (1) Pass ornidazole through a 60-mesh sieve, and grind the rest of the auxiliary materials through a 100-mesh sieve.
[0072] (2) After mixing the ornidazole raw material and the internally added material evenly, dry granulation to prepare 20-mesh granules.
[0073] (3) After the uniformity of mixing and the content are tested to be qualified, it can be obtained by pressing into tablets, and the theoretical quantity is 1000 tablets.
Embodiment 3
[0075] An ornidazole orally disintegrating tablet adopts powder direct compression and is made of components comprising the following weight percentages:
[0076] Ornidazole 250g (50%),
[0077] Crospovidone 90g (18%),
[0078] Mannitol 150g (30%),
[0079] Calcium stearate 2.5g (0.5%),
[0080] Anhydrous colloidal silicon dioxide 7.5g (1.5%);
[0081] The preparation method of ornidazole orally disintegrating tablet in the present embodiment, comprises the steps:
[0082] (1) Pass ornidazole through a 60-mesh sieve, and grind the rest of the auxiliary materials through a 100-mesh sieve.
[0083] (2) Mix the ornidazole raw material and the internally added material evenly.
[0084] (3) After the uniformity of mixing and the content are tested to be qualified, it can be obtained by pressing into tablets, and the theoretical quantity is 1000 tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com