Method for enhancing expression of P-selectin in vascular endothelial cells
A technology of vascular endothelium and P-selectin, which is applied in the fields of biochemical equipment and methods, measurement/inspection of microorganisms, electric/wave energy processing enzymes, etc., can solve the problems of lack of technology, shortage, and ineffective homing of stem cells, etc. To achieve the effect of simple operation and remarkable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
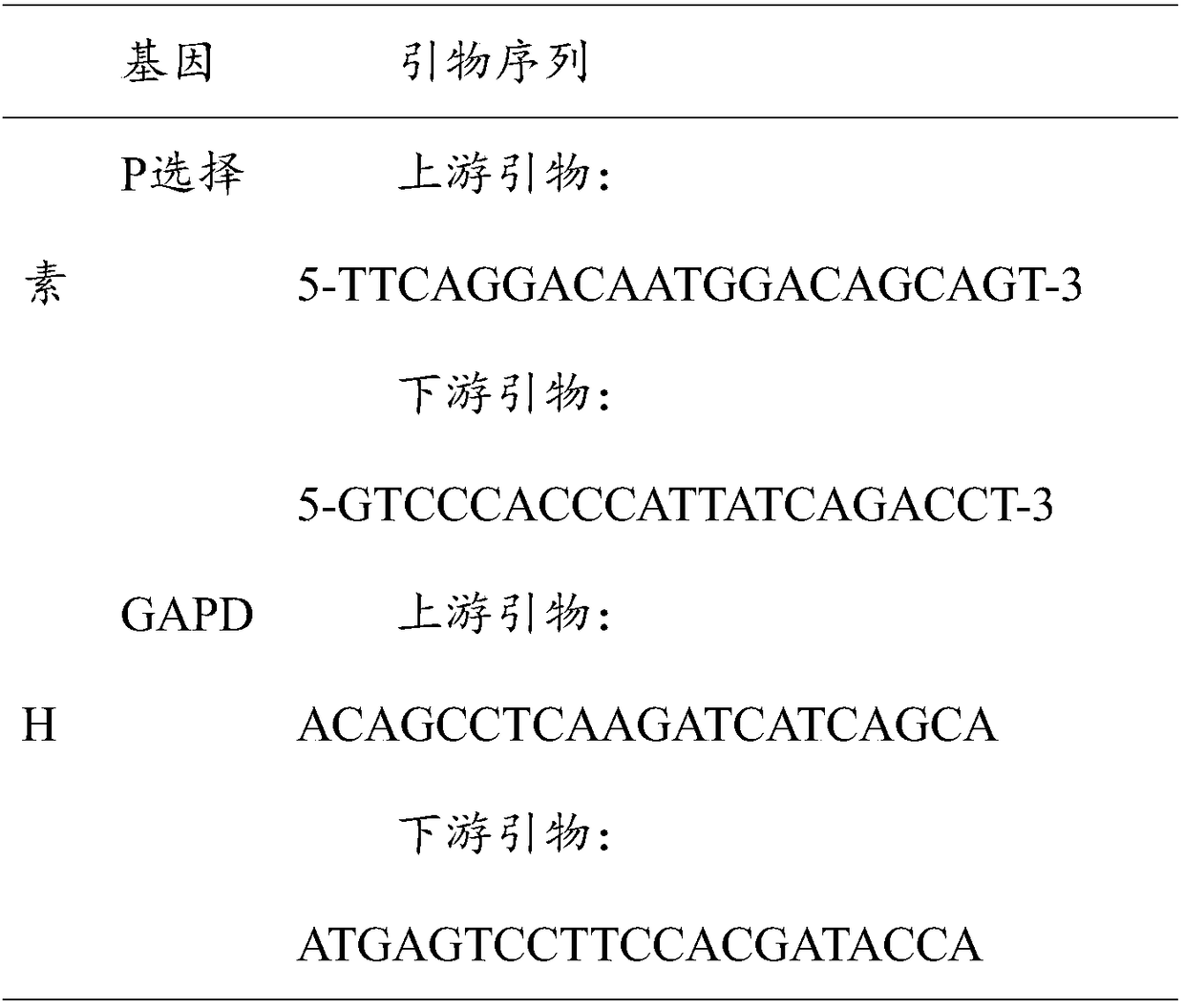
[0017] 1) Take out the cryopreservation tube of primary human cardiac microvascular endothelial cells (HCMEC) from liquid nitrogen, melt it quickly in a 37°C water bath, transfer it to a culture bottle coated with L-polylysine, and put a certain amount of endothelial The cell culture medium was cultivated in a 37°C, 5% carbon dioxide incubator, the medium was changed every 2-3 days, and the cells were passaged once every 4-5 days. The cells were digested with trypsin, and the fifth generation cells were used in the experiment.
[0018] 2) After digesting and centrifuging the 5th generation HCMEC cells, resuspend them with 10% complete culture medium and transplant them into a culture dish; sterilize the shock wave probe, set the parameters to 2.5bar, 10Hz, and use shock waves to act on the cells 200 times , the control group was the fifth generation HCMEC cells without shock wave treatment, and the shock wave probe was selected from the Swiss EMS company.
[0019] 3) The cells...
Embodiment 2
[0025] 1) Take out the cryopreservation tube of primary human cardiac microvascular endothelial cells (HCMEC) from liquid nitrogen, melt it quickly in a 37°C water bath, transfer it to a culture bottle coated with L-polylysine, and put a certain amount of endothelial The cell culture medium was cultured in a 37°C, 5% carbon dioxide incubator, the medium was changed every 2-3 days, and the cells were passaged once every 4-5 days. The cells were digested with trypsin; the fifth passage cells were used in the experiment.
[0026] 2) After digesting and centrifuging the 5th generation HCMEC cells, resuspend them with 10% complete culture medium and transplant them into a petri dish; sterilize the shock wave probe, set the parameters to 1.5bar, 10Hz, and use shock waves to act on the cells 200 times , the control group was the fifth passage HCMEC cells without shock wave treatment.
[0027] 3) The cells subjected to the shock wave treatment were cultured for 60 hours for RNA isolat...
Embodiment 3
[0030] 1) Take out the cryopreservation tube of primary human cardiac microvascular endothelial cells (HCMEC) from liquid nitrogen, melt it quickly in a 37°C water bath, transfer it to a culture bottle coated with L-polylysine, and put a certain amount of endothelial The cell culture medium was cultured in a 37°C, 5% carbon dioxide incubator, the medium was changed every 2-3 days, and the cells were passaged once every 4-5 days. The cells were digested with trypsin; the fifth passage cells were used in the experiment.
[0031] 2) After digesting and centrifuging the 5th generation HCMEC cells, resuspend them with 10% complete culture medium and transplant them into a petri dish; sterilize the shock wave probe, set the parameters to 2.0bar, 10Hz, and use shock waves to act on the cells 200 times , the control group was the fifth passage HCMEC cells without shock wave treatment.
[0032] 3) The cells subjected to the shock wave treatment were cultured for 60 hours for RNA isolat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com